Here’s another abstract dealing with TXA. But this one deals with the classic CRASH-2 use for patients with major bleeding. The original patient showed that TXA improves survival if given within 3 hours of injury. More and more prehospital units (particularly aeromedical services) have been administering TXA enroute to the trauma center to ensure that this drug is given as early as possible.
Many of these same services carry packed cells (or in rare cases, whole blood) so that proper resuscitation can be started while enroute as well. A multicenter group led by the University of Pittsburgh evaluated the utility of giving both TXA and blood during prehospital transport.
Their study summarizes some of the results of the Study of Tranexamic Acid During Air and Ground Medical Prehospital Transport Trial (STAAMP Trial). This study ran from 2015 to 2019 and randomized patients to receive either TXA or placebo during air or ground transport to a trauma center. It included blunt or penetrating patients at risk for hemorrhage within 2 hours of injury who were either hypotensive or tachycardic. Outcome measures included 30-day mortality, 24-hour mortality, and a host of complications.
This abstract outlines a secondary analysis that retrospectively reviewed the impact of using prehospital packed red cells (pRBC) in addition to the TXA/placebo during transport.
Here are the factoids:
- There were 763 patients in total, broken down as follows
- TXA only – 350
- pRBC only – 35
- TXA + pRBC – 22
- Neither – 356
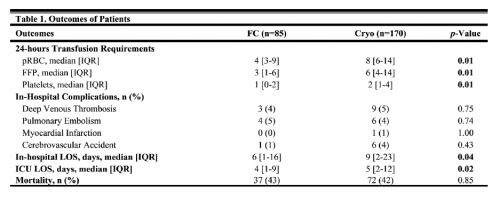
- Patients who received blood with or without TXA were more severely injured with ISS 22 vs 10-12 in the non-pRBC groups
- Mortality was higher in the pRBC (23%) and TXA+pRBC groups (29%)
- TXA alone did not decrease mortality
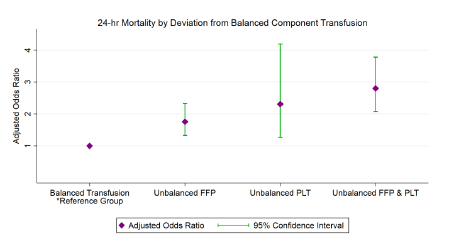
- TXA + pRBC resulted in a 46% reduction in 30-day mortality but not at 24 hours
- packed cells alone decreased 24-hour mortality by 47%
The authors concluded basically what was stated in the results: short term mortality was decreased by pRBC alone, and 30-day mortality with TXA + pRBC. They recommended further work to elucidate the mechanisms involved.
Bottom line: This abstract may also suffer from the “low numbers” syndrome I’ve written about so many times before. The conclusions are based on two small groups that make up only 7% of the entire study group. And these are the two groups with more than double the ISS of the rest of the patients. The authors used some sophisticated statistics to test their hypotheses, and they will need to explain how and why they are appropriate for this analysis. Nevertheless, the mortalities in the blood groups number only in the single digits, so I worry about these statistics.
Here are my questions for the authors and presenter:
- How do you reconcile the significantly higher ISS in the two (very small) groups who got blood? How might this skew your conclusions regarding mortality? Couldn’t the TXA just be superfluous?
- How confident are you with the statistical analysis? Could the results be a sampling error given that red cells were given to only 7% of the overall study group?
- I am having a difficult time understanding the conclusion that mortality was reduced in the blood groups. Specifically, it is stated that 24-hour mortality is reduced by 47% in the blood-only group. But the mortality is 14% (5 patients)! Reduced 47% from what? I don’t see any other numbers to compare with in the table. Confusing!
Obviously, there must be more information that was not listed in the abstract. Can’t wait to see it!
Reference: PREHOSPITAL SYNERGY: TRANEXAMIC ACID AND BLOOD TRANSFUSION IN PATIENTS AT RISK FOR HEMORRHAGE. EAST 35th ASA, oral abstract #39.
Reference: PREHOSPITAL SYNERGY: TRANEXAMIC ACID AND BLOOD TRANSFUSION IN PATIENTS AT RISK FOR HEMORRHAGE. EAST 35th ASA, oral abstract #39.