Uncontrolled bleeding is the bane of trauma professionals everywhere. Early in a resuscitation, we focus on identifying potential sources. We’ve developed numerous techniques for plugging them up. And we have processes in place to replace the blood that’s been lost.
Unfortunately, blood products are a perishable item. Packed red blood cells have a typical shelf-life of 42 days. Whole blood lasts only 21-35 days. Plasma is only suitable for up to five days once thawed. However, it can be frozen and used only when needed.
Platelets are another short-lifespan product, typically lasting only five days. This is a major reason for the relative lack of availability, especially at smaller hospitals. Unfortunately, freezing them or attempting cold storage renders them less active. For this reason, the platelet shortage persists.
As you know, platelets are fragments of cells produced by the bone marrow that have a major function in hemostasis. They bind to injured surfaces of disrupted blood vessels. Seconds later, they become activated and begin to clump with other platelets. They also release factors that result in fibrin deposition, creating a clot that helps stop bleeding.
Researchers have been trying to develop artificial blood substitutes for decades. I remember reading about rat experiments using these products in the 1980s. Unfortunately, they remain experimental to this day.
I found a recent article describing recent work on artificial platelets that piqued my interest. It was published by the biomedical engineering groups at North Carolina State University and UNC Chapel Hill. They used nanoparticles made of an ultrasoft microgel that were similar in size and shape to natural platelets. Fibrin-binding antibody fragments were embedded on the surface. These were selected to target only activated fibrin and not circulating fibrinogen.
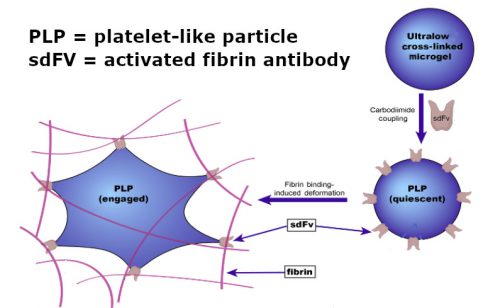
Source: Science Translational Medicine
The groups devised a rat and pig trauma model by creating a liver laceration and then infusing varying doses of the artificial platelets (AP). Postmortem analysis of the wounds showed:
- The APs did home in on the injured sites and were found in the injured areas
- There was increased fibrin deposition at the wound site when compared to saline controls
- Less bleeding was seen in the animals that received the APs vs saline
- No significant deposition of APs was found in other tissues
- The APs were excreted in the urine of the animals
Bottom line: This is very exciting, if preliminary, work. These artificial platelets are relatively easy to produce and can be frozen or stored at room temperature for extended periods. They appear harmless to the animals and decrease bleeding from the liver injury.
I am still somewhat cautious in my assessment. This same excitement was present 40 years ago in the early years of artificial hemoglobin solutions. And look where we are now. But, fingers crossed, there may be a solution to our chronic platelet shortage at some point in the future.
Reference: Ultrasoft platelet-like particles stop bleeding in rodent and porcine models of trauma. Sci Transl Med. 2024 Apr 10;16(742):eadi4490. doi: 10.1126/scitranslmed.adi4490. Epub 2024 Apr 10. PMID: 38598613.