Early resuscitation, particularly with blood products in patients with hemorrhage, is literally a lifesaver. As each minute ticks by, survival slowly diminishes. To facilitate this, massive transfusion protocols (MTP) have been designed to rapidly deliver sizable quantities of blood products to the trauma resuscitation bay.
One of the recurring issues I see at trauma centers is the lack of a reliable way of activating the MTP. Many centers publish what I consider “psychic criteria.” These promote criteria that involve the amount of blood loss over four or twenty-four hours. Who even knows?
Delays in activating the MTP frequently occur because no one thinks about it when a critically injured patient arrives. All of the trauma professionals are busy with the patient and are rudely surprised when they ask for the first unit of blood.
Objective MTP activation criteria have been developed and are well-supported by the literature. The ABC score and the shock index are two of the more common methods. Both are based on observations made upon patient arrival (and possibly before if a prehospital report is received).
The ABC score uses four criteria:
- Heart rate > 120
- Systolic blood pressure < 90
- FAST positive
- Penetrating mechanism
If any two of these are present, there is a 50% chance that massive transfusion is warranted.
The Shock Index (SI) uses the initial vital signs to perform a quick and dirty calculation by dividing the heart rate by the systolic blood pressure. A score greater than or equal to one predicts at least a 2x higher need for blood. Of the two, SI is more easily calculated and gives a marginally more accurate result.
But what about children? The ABC score was evaluated in pediatric patients and was found to be much less sensitive than in adults. Combining the ABC score with an age-adjusted Shock Index improved the accuracy only slightly. This was named the ABC-S score.
Several adult and pediatric trauma centers in the Denver area collaborated to test a new score using the ABC-S score in combination with serum lactate and base deficit. This was termed the ABC-D score. Clever.
Here are the factoids:
- A retrospective review of patients aged 1-18 from a single trauma registry who had received a blood transfusion during their initial care
- The study included 211 children, of whom 66 required massive transfusion
- The three methods listed above were compared, and the ABC-D score was found to be the most predictive of MTP
- ABC-D was 77% sensitive and 79% specific
- The authors showed that the accuracy and balance between sensitivity and specificity improved for each point increase in the ABC-D score.
- They concluded that ABC-D may be a useful tool to expedite the delivery of blood products during a trauma resuscitation.
Bottom line: Hmm. The system that they developed and the analysis of their experience appears to be sound. But unfortunately, it fails the practicality test. Here’s the sticking point. How long does it take to obtain that initial blood specimen, send it to your lab, and then return stat results to your trauma bay? Once you receive the results, you then activate the MTP and wait another 5-10 minutes for the first cooler to arrive!
That’s an awful long time to wait for blood while you watch a child hemorrhaging in front of you. So what to do? For now, use one of the existing systems to make a rapid decision. And always err on the side of activation. You can always send the blood back if you don’t need it!
Reference: The ABC-D score improves the sensitivity in predicting need for massive transfusion in pediatric trauma patients. J Pediatr Surg. 2020 Feb;55(2):331-334. doi: 10.1016/j.jpedsurg.2019.10.008. Epub 2019 Nov 1. PMID: 31718872.


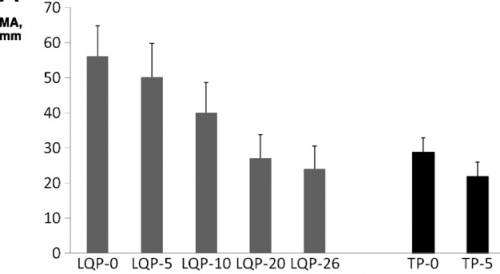 (TEG MA for liquid (LQP) and thawed (TP) plasma
(TEG MA for liquid (LQP) and thawed (TP) plasma