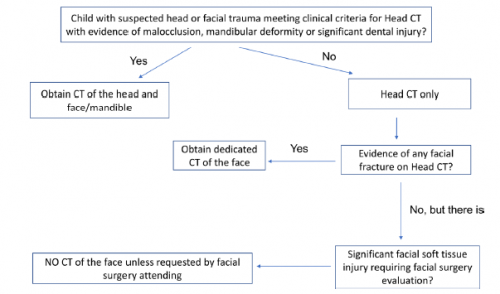
A bucket-handle injury is a relatively uncommon complication of blunt trauma to the abdomen. It only occurs in a few percent of patients, but is much more likely if they have a seat belt sign. The basic pathology is that the bowel mesentery (small bowel of sigmoid colon) gets pulled away from the intestinal wall.
This injury is problematic because it may take a few days for the bowel itself to die and perforate. Patients with no other injuries could potentially be discharged from the hospital before they become overtly symptomatic, leading to delayed treatment.
Here’s an image from my personal collection with not one, but four bucket-handle injuries.
Typical patients with suspected blunt intestinal injury are observed with good serial exams and a daily WBC count. If this begins to rise after 24 hours, there is a reasonable chance that this injury is present.
CT scan has not really been that reliable in past studies. There may be some “dirty mesentery”, which is contused and has a hematoma within it. But without a more convincing exam, it is difficult to convince yourself to operate immediately on these patients.
A paper was published by a group of radiologists at Duke University. It appears to be a case report disguised as a descriptive paper. It looks like they picked a few known bucket-handle injuries from their institution and back-correlated them with CT findings.
The authors called out the usual culprits:
- Fluid between loops of bowel
- Active bleeding in the mesentery
- Bowel wall perfusion defects
But they also noted that traumatic abdominal wall hernias were highly associated with seat belt sign as well. These are rare, but should bring intestinal injury to mind when seen.
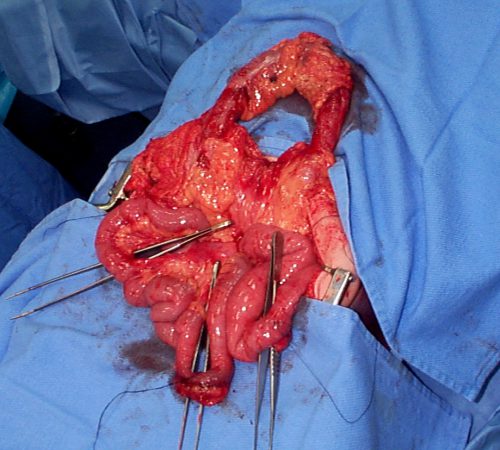
With newer scanners, radiologists are better able to detect subtle areas of hypoperfusion as well. This is a fairly good indicator of injury, especially when adjacent bowel appears normally perfused. Here are two examples. The black arrows denote active extravasation, and the white ones an area of hypoperfusion.
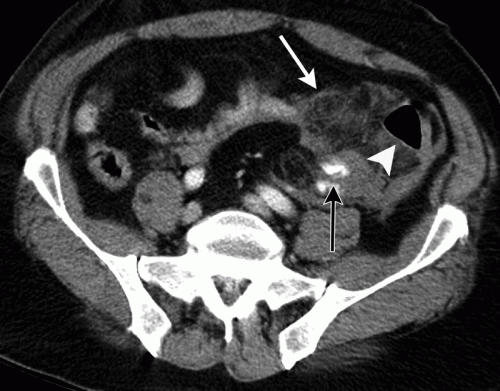
The authors add bowel wall hypoperfusion as another finding that may point to a bucket-handle type injury.
A recent paper demonstrates the value of the current generation of high-quality scanners. A collection of California and Denver centers implemented a multicenter, prospective, observational study of patients with seat belt signs. The developed a list of positive findings, which included:
- abdominal wall soft tissue contusion (radiographic seat belt sign)
- free peritoneal fluid
- bowel wall thickening
- mesenteric stranding
- mesenteric hematoma
- bowel dilation
- pneumatosis
- pneumoperitoneum
A total of 754 patients with visible seat belt sign were enrolled and all went to CT scan. Any of the findings listed above were associated with a statistically significant likelihood of hollow viscus injury. The highest likelihood was associated with:
- free peritoneal fluid – 42x more likely
- bowel dilation – 21x
- free fluid with no solid organ injury – 20x
- bowel wall thickening – 19x
- radiographic seat belt sign – 3x
Any of the radiographic findings strongly suggested that an injury could be present. However, if none were present, it was very unlikely that there were any significant injuries. The authors suggested that if such patients had no other injuries requiring hospitalization, they could potentially be discharged home. However, those patients should be counseled to return for evaluation immediately if they have any change in their abdominal or systemic status.
Bottom line: Some patients with a visible seat belt sign might be eligible for discharge from the ED if they have a totally negative abdominal CT and no other injuries requiring hospitalization. If they have any finding, they should be admitted for observation.
If your patient has an unconcerning exam and any of the findings listed above, perform serial exams and get a WBC the next morning. If the exam worsens, operate. If the WBC rises, consider laparoscopy to see if you need to make a bigger incision. And if you see any evidence of hypoperfused bowel, consider laparoscopy right away.
References:
- Excluding Hollow Viscus Injury for Abdominal Seat Belt Sign Using Computed Tomography. JAMA Surg. 2022 Sep 1;157(9):771-778. doi: 10.1001/jamasurg.2022.2770. PMID: 35830194; PMCID: PMC9280606.
- CT findings of traumatic bucket-handle mesenteric injuries. Am J Radiol 209:W360-@364, 2017.
- Multidetector CT of blunt abdominal trauma. Radiology 265(3):678–693, 2012.