Let’s kick off my reviews of AAST 2020 abstracts with a paper on the results of recent advances in hemorrhage control. Over the past 10+ years we have seen the following new (and old) tools move into more widespread use:
- Massive transfusion protocol (MTP) with a goal of 1:1 ratios of red cells to plasma
- Availability of liquid plasma for more rapid use in the MTP
- Addition of tranexamic acid (TXA) to resuscitation
- Resurgence of tourniquet use by prehospital providers
- Adoption of REBOA and TEG
- Transfusion with whole blood
The authors analyzed their experience after serially introducing these tools to their resuscitation strategies, and studied their impact on overall mortality.
They retrospectively reviewed the experience over a 12 year period at their large Level I trauma center. Here are the factoids:
- The reviewed a total of 824 MTP events. To put this into perspective from a volume standpoint, this is a little over one MTP activation per week.
- Patients were primarily young (median age 31), male (81%), with a penetrating mechanism (68%). Median ISS was 25
- Prehospital times were significantly longer at the end of the study, but the authors state that there was no correlation with an increase in in-hospital mortality
- During the entire study, overall mortality ranged from 38% to 57%, and logistic regression did not identify an effect from any of the interventions
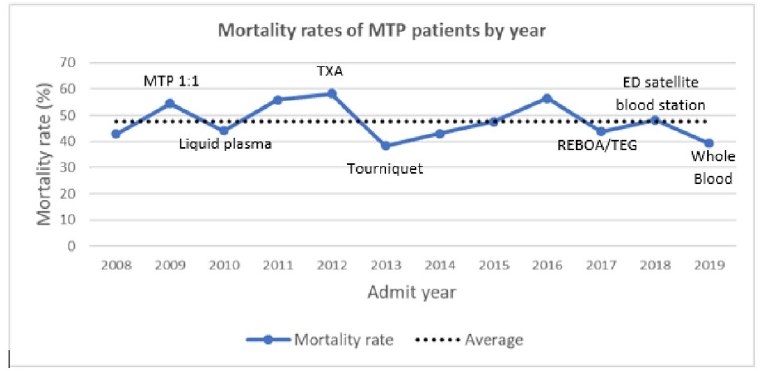
The authors concluded that their mortality rates have not improved despite all of the advancements we have added over the past decade. They suggest that future efforts should attempt to move targeted hemorrhage control backwards in time, out of the ED and toward to injury scene.
Here are my comments: This is an interesting and simple-appearing study. Overall, the authors didn’t really show that any of our “modern” resuscitation interventions did much for their patients at all. There was a suggestion that tourniquet implementation and use of whole blood tended toward improving things.
But don’t be fooled by simplicity. There are many, many factors that enter into whether an individual patient lives or dies. When you fail to see a significant result in a study, first look at the methods and tools used for measurement. Are they powerful enough to discern changes? Do they cover enough of the factors that promote survival, not just our resuscitative advances? Or is the tool looking at the wrong things?
One big difference at this center is the sheer volume of penetrating trauma. This could have a major impact on survival, and may be very different from the experience of most centers that have predominantly blunt injury mechanisms.
And some questions for the authors:
- What exactly is your definition of mortality? Made it out of the ED? Lived twenty four hours? Thirty days? This makes a big difference in how you look at the results.
- Since you have only about one MTP event per week, do you think your numbers are large enough to actually detect a mortality difference?
- Did you consider looking at your unexpected survivors to see if there were any common threads in their care that might have made the difference? Maybe some of our resuscitative advances do make a difference, but only in specific subsets of patients.
- Can you speculate about the reasons for longer prehospital times, and the impact on mortality?
- How would you recommend pushing hemorrhage control back toward the scene? New tools for prehospital providers? More advanced providers in the rigs? This is an intriguing concept and it would be interesting to hear your thoughts.
This is a thought provoking paper that questions our assumptions about our time-honored resuscitation tools. I look forward to hearing it live next month!
Reference: After 800 MTP events, mortality due to hemorrhagic shock remains higha nd unchanged despite several hemorrhage control advancements; is it time to move the pendulum? AAST 2020 Oral Abstract #1.