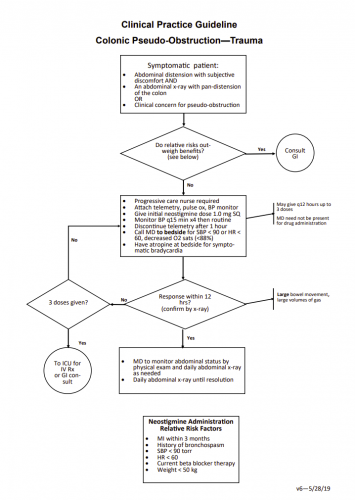
Nausea and vomiting are common problems in trauma patients, particularly those in a trauma activation. Inciting factors include pain, full stomach from food eaten before the event or blood swallowed after, or reaction to pain medications. For years, trauma professionals reached for the lowly gastric tube to evacuate stomach contents to “solve” the problem.
But how many of you have seen a patient forcefully empty their stomach as soon as the tube touches the oropharynx? And of course, your patient is lying supine, so the vomitus goes straight up, then back down into their airway. And if their mental status is not quite right, they may aspirate, causing even bigger problems.
We’ve had anti-emetic medications for a long time, some more effective than others. Only recently have we begun to rely on these as a first line defense in the trauma resuscitation room. But do they work? Are they safer?
The University Medical Center Utrecht in the Netherlands looked at this problem. They changed their policy from inserting a gastric tube to administering anti-emetics at the beginning of 2014. They studied their experience for the 6 months before and 6 months after the policy change. They inserted an orogastric (OG) tube preferentially before the switch, and used ondansetron and/or metoclopramide after.
Here are the factoids:
- A total of 1446 trauma patients were admitted during this period. After excluding patients who were intubated or did not complain of nausea, 453 were analyzed (30%)
- 20% of patients who had an OG tube placed vomited vs only 3% receiving medication (significant)
- After therapy, 14% of patients receiving an OG were still nauseated vs only 2% getting meds (also significant)
- 3 patients vomited and aspirated after OG placement, and 1 developed a pneumonia. 2 patients became bradycardic and med administration, and one developed QT-prolongation
Bottom line: This is a relatively small, retrospective study. Furthermore, the choice of gastric tube route (oral) is a setup for gagging and vomiting. Nasogastric tubes are a bit less noxious, but can’t be inserted in all patients (see next week’s post). Even so, the use of anti-emetics in trauma patients complaining of nausea seems like the kinder, gentler way to go.
Which drug to use? Previous studies have shown that ondansetron 4mg is as effective as 8mg, and that this drug is about equally as effective as metoclopramide. There is also some evidence that giving both is more effective than just giving one.
Gastric tubes are still important, particularly in the comatose patient. But since these patients are at risk for cribriform plate injury, only the oral route should be used.
Reference: Analysis of two treatment modalities for the prevention of vomiting after trauma: orogastric tube or anti-emetics. Injury (accepted manuscript, in press) online 8 July 2017.