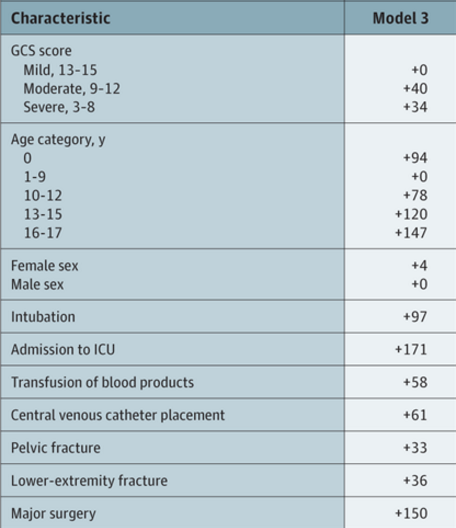
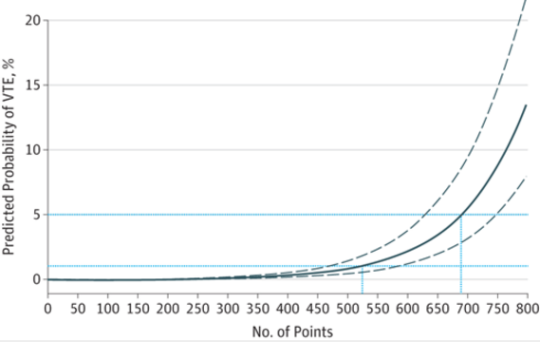
Venous thromboembolism (VTE) is a potential problem for all hospitalized patients, and traumatic injury is yet an additional risk factor for its occurrence. Most trauma centers have some kind of risk assessment tool to help the tailor their chemoprophylaxis regimen to patients most at risk. But far fewer have adopted the use of screening ultrasounds to monitor for new onset VTE that would dictate conversion to therapeutic treatment.
Unfortunately, in the US, the Centers for Medicare and Medicaid Services (CMS) has deemed VTE as a “never” event and penalizes hospitals when they report it. One of the unintentional consequences of this (or is it?) is that hospitals may then pressure trauma programs to avoid surveillance in order to “make the numbers look better.” Remember Law X from Samuel Shem’s House of God?
X. If you don’t take a temperature, you can’t find a fever.
Similarly, if you don’t do a duplex screen, you probably won’t detect VTE. Now granted. some patients develop classic symptoms like edema, pain, and tenderness. But not that many.
But is this wise? My contention has been that if the patient doesn’t develop symptoms that catch your attention, yet they develop VTE that you don’t know about, they are at risk for more serious complications like pulmonary embolism (PE). And you are blithely unaware.
The trauma group at Intermountain Medical Center in Salt Lake City performed an elegant study to determine the impact of screening for VTE in their trauma patients. They performed a prospective, randomized trial on trauma patients admitted over a 30-month period. Patients were included if they were judged to be at moderate to high risk based on their risk assessment profile (RAP) score. Patients were excluded if they were children, had VTE or PE within 6 months prior to hospitalization, or had some type of hypercoagulable state.
Patients were sequentially randomized to no duplex screening vs screening on days 1, 3, 7, and then weekly thereafter. The primary outcome measure was PE during the hospital stay. Secondary outcomes consisted of a number of factors relating to development of DVT.
Here are the factoids:
- Nearly two thousand patients were enrolled, with about 995 patients in each group and no differences in demographics
- The ultrasound group had significantly more below-knee (124 vs 8) and above knee (19 vs 8) DVT identified (no surprise there)
- The ultrasound group had significantly fewer pulmonary emboli than the no ultrasound group (1 vs 9) (lots of surprise here!)
- Mortality was similar during the hospital stay and for 90 days after
Bottom line: If you look for it, you will find it! This is the definition of surveillance bias. But in in this study, looking for clots in the legs may also decrease the number of patients who develop symptomatic pulmonary embolism. How could this be?
There are a few possibilities. The majority of DVT found in the surveillance group were located distally. Although there is some uncertainty as to how likely these are to embolize, it is probably very low. So let’s ignore them for now and assume that only the proximal clots might embolize.
This leaves an extra 11 DVT found in the surveillance group over and above the no-ultrasound group. Despite that, the surveillance group had only one PE vs 9 in the no-ultrasound group!
Another explanation was that the ultrasound guided changes in management, shifting to management to therapeutic drug dosing. The authors did not find a significant difference between the use of therapeutic vs prophylactic dosing between the groups. But there was a difference. Although the overall study was well-powered, there really weren’t enough numbers to show whether there was a true difference in therapeutic dosing. Fourteen patients in the ultrasound group got therapeutic anticoagulation compared to only 4 in the no-surveillance group. I think this is the actual reason.
Overall, this is a well-designed and well-executed study that shows why taking the Ron Popeil approach to DVT prophylaxis (“set it and forget it”) doesn’t work. Patients do occasionally develop proximal DVT on standard chemoprophylaxis (and frequently develop distal DVT), but it doesn’t always result in obvious signs and symptoms. This study shows that if you don’t look for it, you may not know until they suddenly develop chest pain, air hunger, and worse! So consider carefully if your practice guideline doesn’t yet include surveillance.
Reference: Trauma Patients at Risk for Venous Thromboembolism who Undergo Routine Duplex Ultrasound Screening Experience Fewer Pulmonary Emboli: A Prospective Randomized Trial. J Trauma, publish ahead of print, Publish Ahead of Print. DOI: 10.1097/TA.0000000000003104, February 4, 2021.