In theory, tiered trauma centers should allow patients with lesser injuries to be treated at lower levels and more severe trauma at higher-level centers. This parallels the resource availability at those centers. In reality, many patients with injuries that seem complex (solid organ, children, and TBI) are transferred due to a “lack of comfort” in taking care of them or the perception that they may deteriorate quickly.
The truth is that many, if not most, of these patients are discharged shortly after transfer to the higher-level center, with minimal intervention. This burdens the trauma system in several ways. First, trauma professionals at the lower-level centers slowly lose their skills and comfort in taking care of these patients. The prehospital system is already plagued with low resources and a shortage of personnel. Using one of the only ambulances in a rural area for transport to a distant center takes it out of the community, potentially putting the area population at risk for delayed care.
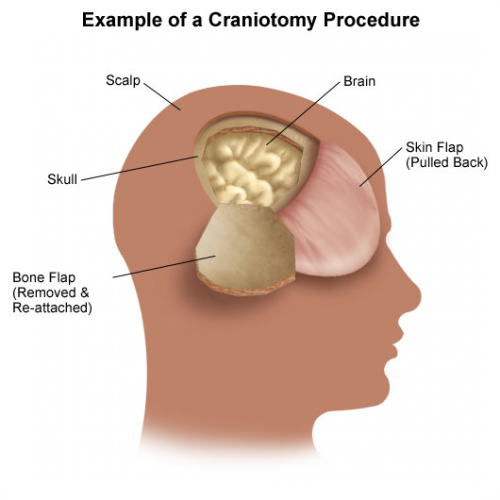
The University of Arizona at Tucson group performed another TQIP study to highlight this problem. They performed a four-year retrospective analysis of transfer data in patients with isolated TBI with intracranial hemorrhage. They observed the number of transferred patients who required CT scans, ICP monitoring or craniotomy/craniectomy, length of stay, and mortality.
Here are the factoids:
- Of the nearly 120,000 patients with isolated TBI at Level I and II centers, 45% were transferred from other centers
- Most patients had GCS 14-15 on arrival, but 10% had GCS 8
- CT was performed in 58%, and another repeat CT in 4%
- Four percent underwent ICP monitoring, and 12.5% had a crani
- Mortality was 6.5%
- Median length of stay was two days, with a range of 1-5
- 18% were discharged within 24 hours, and 39% within 48 hours
The authors concluded that, while half of isolated TBI patients were treated at high-level trauma centers, one-third were discharged home within 48 hours with no intervention other than a CT scan. They recommend systemwide guidelines to improve resource utilization.
Bottom line: This straightforward analysis highlights one of the most significant issues facing trauma systems: unnecessary transfer. For decades, Level I and II centers were convenient and always available for transfer, even if the indications were questionable. Then COVID came along and changed everything.
Now, resources are tight everywhere. EMS is underpaid and under-reimbursed, and personnel are difficult to recruit. Hospital personnel of all types face low staffing levels, making it more stressful to provide the level of care we are accustomed to. Skilled nursing facilities, rehab centers, and other outpatient care settings face the same problems.
This has created a domino effect, where the lower-level centers want to transfer a patient but can’t find a bed at the higher-level centers. When they do, it takes forever to get them transported. Then, the higher-level centers can’t discharge them if they need any level of care other than home care.
There are many pieces to this puzzle, but this abstract clearly outlines one of them. Lower-level centers are transferring some patients who could actually be admitted to them. Several reasons may be given, but it typically boils down to the surgeons or the hospitalists not being “comfortable” with certain patients or worrying that they could deteriorate.
This paper does not tease out what kind of isolated TBI the patients had, and I recommend they do. There is a big difference in patients with a subarachnoid hemorrhage (SAH) vs. those with an epidural. The number of patients with SAH is far greater, and the vast majority can go home after a brief (or no) observation. The likelihood of deterioration in patients not on blood thinners is nearly zero.
State trauma systems and higher-level trauma centers should work with their Level III and IV partners to adopt consistent practice guidelines and protocols to stratify these patients to identify those at low risk. The higher-level centers should provide education to help their referral partners develop a baseline comfort level with these patients. This is the only way we can begin to realign the levels of trauma centers with the levels of care needed by our patients.
Reference: Endless highways: the burden of transferred traumatic brain injury patients in the United States. EAST 2024, Podium paper #42.