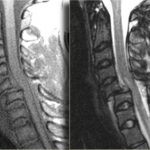
Time for a little philosophy today. There seem to be two camps in the world of initial diagnostic testing for trauma: selective scanning vs scan everything. I admit that I am one of the former. Yes, the more tests you do, the more things you will find. Some will be red herrings. Some may be true positives, but are they important? Here’s the key question:
“If a tree falls in a forest and no one is around, does it make a sound?”
There is a clinical corollary to this question in the field of trauma:
“If an injury exists but no one diagnoses it, does it make a difference (if there would be no change in treatment)?”
Here’s an example. On occasion, my colleagues want to order diagnostic studies that won’t make any clinical difference, in my opinion. A prime example is getting a chest CT after a simple blunt assault. A plain chest xray is routine, and if injuries are seen or the physical exam points to certain diagnoses, appropriate interventions should be taken. But adding a chest CT does not help. Nothing more than the usual pain management, pulmonary toilet, and an occasional chest tube will be needed, and those can be determined without the CT.
Trauma professionals need to realize that we don’t need to know absolutely every diagnosis that a patient has. Ones that need no treatment are of academic interest only, and can lead to accidental injury if we look for them too hard (radiation exposure, contrast reaction, extravasation into soft tissues to name a few). This is how we get started on the path to “defensive medicine.”
Bottom line: Think hard about every test you order. Consider what you are looking for, what you might find, and if it will change your management in any way. If it could, go ahead. But always consider the benefits versus the potential risks, or what I call the “juice to squeeze ratio.”
Tomorrow I’ll look at some of the “scan all” vs “scan selectively” literature. Which camp are you in?
References:
- George Berkeley, A Treatise Concerning the Principles of Human Knowledge, 1734, section 45.
- paraphrased by William Fossett, Natural States, 1754.