I’ve got more blood transfusion stuff for you today! This one isn’t exactly MTP, but it does deal with patients suffering from blood loss. Major trauma patients requiring transfusion are losing all of their clotting factors in addition to red cells and platelets. Some factors can be replaced by giving plasma. However, clotting factor I (fibrinogen) is not one of them. For that reason, most MTPs include administration of cryoprecipitate to replace it at key points in the algorithm.
However, fibrinogen replacement actually is available in two forms, cryoprecipitate (cryo) and fibrinogen concentrate (FC). These two forms have very different properties and costs. The authors from the University of Arizona Tucson massaged two years worth of data from the TQIP database. They included all trauma patients who received either cryo or FC, except for those with known clotting issues from bleeding disorders, liver disease, or anticoagulants they were taking. They analyzed differences in mortality, complications, transfusion requirements, and length of stay.
Here are the factoids:
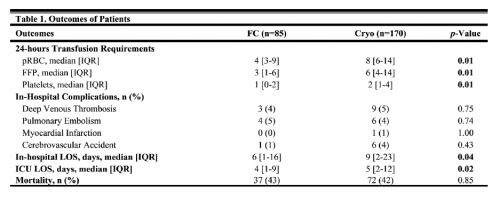
- A total of 85 patients received FC and 170 received cryo (?)
- Blood product usage (red cells, plasma, and platelets) was less in the fibrinogen concentrate group (see table)
- ICU and hospital lengths of stay were less in the FC group (see table)
- There was no difference in complications between the two groups
The authors concluded that use of fibrinogen concentrate was associated with improvement in the outcomes they measured. They recommended that further studies be done to evaluate use of FC.
Bottom line: This study suggests that use of fibrinogen concentrate may be better than cryoprecipitate. But there are a lot of things to think about before jumping to conclusions. First, remember that this is an association paper. It cannot detect cause and effect. Blood component usage and LOS were indeed less in the FC group. But how many hundreds of other variables factor into those outcomes: specific injury pattern, ISS, body temperature, resuscitation balance, time to OR, and product availability are just a few. These and a host of others cannot be controlled easily in a study using TQIP data alone.
Next, look at the number of units of blood products given as listed in the table above. These are the average number of units given within 24 hours! These are relatively low numbers, and below what most would consider a “massive transfusion” threshold. Most of the time, we wouldn’t even think to measure fibrinogen in patients with such low blood replacement needs. Perhaps we should?
And finally, I have questions about the low number of subjects in the study. There are only 255 patients analyzed, and propensity score matching was used. This implies that overall numbers were low and that several covariates had to be considered. It seems like more subjects should have been available in the tens of thousands of patients submitted in two years to TQIP
Here are my questions for the authors and presenters:
- Please review your statistical analysis with the audience. Why is the number of subjects so low? What did you control for in your propensity score matching and how did this impact your n?
- Comment on the low numbers of blood products given. Do you think that patients who have such a relatively low transfusion requirement even needed a fibrinogen supplement?
- Did you consider performing this analysis on patients who underwent massive transfusion? The effect might have been more pronounced in that group.
- Since fibrinogen concentrate costs about 3x what cryo does, how would this factor into the math involved in deciding to use FC?
This study raises a lot of interesting questions. I hope the authors incorporate the answers into their presentation!
Reference: FIBRINOGEN SUPPLEMENTATION FOR TRAUMA PATIENTS: SHOULD YOU CHOOSE FIBRINOGEN CONCENTRATE OVER CRYOPRECIPITATE? EAST 25th ASA, oral abstract #3.