Forty years ago, the presumption was that the best way to intubate a trauma patient was to take them to a fully equipped operating room and have an anesthesiologist perform it. Then, a few years later, we finally figured out it could be done in the emergency department. The key to doing it safely was that the trauma bay needed to look like an OR, with appropriate airway equipment, lights, and drugs. And you had to ensure that your intubator had sufficient skills.
But we are all too familiar with one subset of trauma patients much more sensitive to the intubation process: those who are bleeding and in shock. They are desperately compensating to attempt to maintain their vital signs as much as they can with their sympathetic tone. Unfortunately, the intubation process and the drugs we use can eliminate this reflex and lead to immediate hemodynamic collapse.
The trauma group at Johns Hopkins postulated that intubation in the ED could lead to worse outcomes in this particular group of patients. They analyzed three years of data from the National Trauma Data Bank dataset, isolating patients at Level I and Level II trauma centers who underwent immediate hemorrhage control surgery after arrival. Patients who were dead on arrival, intubated for airway concerns, or underwent resuscitative thoracotomy were excluded.
The authors used a regression model to determine any association between intubation and mortality. They also analyzed the usual secondary outcomes (complications [cardiac arrest, ARDS, AKI, sepsis], transfusions, and time in the ED).
Here are the factoids:
- Nearly ten thousand patients at 253 trauma centers met inclusion criteria
- Most patients were men with penetrating injury
- One in five underwent intubation in the ED before their hemorrhage control operation and suffered a 17% mortality rate vs. 7% in the OR intubation group, which was a significant difference
- Median dwell time in the ED was 31 minutes vs. 22 minutes in the OR group
- Transfusion amount was significantly higher in the ED vs. OR group (6 vs. 4 units RBC)
- Rates of all complications were significantly higher in the ED vs. OR groups (except sepsis)
- Overall, cardiac arrest with CPR occurred in 10% of ED vs. 4% OR intubations
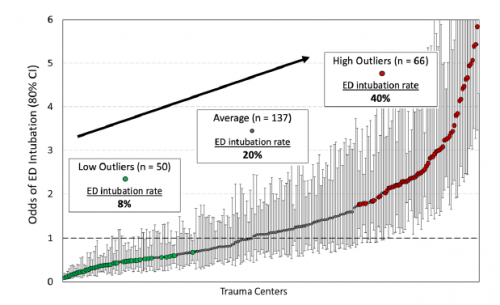
- Centers that had low ED intubation rates generally had significantly lower post-intubation cardiac arrest events than those with higher ED intubation rates.
The authors concluded that ED intubation of patients requiring hemorrhage control was associated with multiple adverse events. They recommended that these patients be taken to the OR, where both intubation and rapid bleeding control can be achieved.
Bottom line: This nice, clean abstract addresses a simple question. Although it uses a large database, the authors focused on a limited number of variables, keeping the analysis uncomplicated.
The abstract paints a clear picture that agrees with the subjective observations of many trauma professionals that intubation in these patients can be dangerous. They found significant increases in mortality and complications in patients intubated in the ED.
Does this mean that the procedure is not being done as well there? Absolutely not! I believe the key is in the ED dwell time data, which shows an average of 9 more minutes spent there for intubation. Previous research has shown how even a few minutes count when it comes to hemorrhage control. This abstract provides some hard numbers that show how important it really is to get to the OR.
Here are my questions and comments for the presenter/authors:
- First, a minor point: how can the “median” GCS be 15? Fifteen is the highest it can go. The median is the number where half the results are higher and half are lower. So if no results can be higher, none can be lower. Does this mean that every one of your 10K patients was wide awake?
- Please explain the figure a little better. Does it just show the mix of low vs. average vs. high ED intubation rates? Or does it go along with the statement that high intubation rate centers have a higher likelihood of cardiac arrest in these patients?
I really enjoyed this abstract and am looking forward to any additional details provided at the presentation.
Reference: EMERGENCY DEPARTMENT VERSUS OPERATING ROOM INTUBATION OF PATIENTS UNDERGOING IMMEDIATE HEMORRHAGE CONTROL SURGERY, EAST 2023 Podium paper #13.