Focused abdominal sonography for trauma (FAST) has been a mainstay of rapid diagnosis for many years. The extended FAST exam (eFAST) adds an examination of the thoracic cavities to the basic exam. The sensitivity and specificity of FAST have mostly been determined. However, there is much less literature outlining the accuracy of the eFAST.
The group at Vanderbilt performed a prospective, observational study on the ability of the eFAST exam to detect pneumothorax specifically. Imaging was performed by a licensed sonographer and read by the attending surgeon and a radiologist as it was completed.
Here are the factoids:
- Only patients receiving chest X-rays, chest ultrasound, and chest CT for confirmation were included in the study, which totaled 1,499
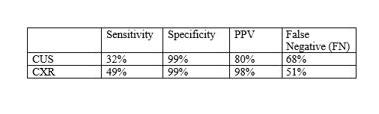
- The statistical analysis was as follows:
- Only 25% of patients had a chest tube placed
- Patients with false negative exams were more likely to have rib fractures and lower oxygen saturation and blood pressure
The authors concluded that chest ultrasound had a low sensitivity and high false negative rate and that many of the negatives eventually required chest tube insertion. They advise that the chest ultrasound should be used alone with caution.
Bottom line: This is an interesting and relatively large study. However, it is at odds with a paper published in 2021 from George Washington University. They published a series of 3,410 patients and found 71% sensitivity, 99% specificity, 87% PPV, and a 2.2% false negative rate.
These are very disparate numbers. However, the GW study was retrospective and included both pneumothorax and fluid in the abdomen or chest, whereas this abstract looked strictly at pneumothorax and was prospective.
Both studies are interesting on their own, and the presenter of this abstract will need to explain why the results are so different. The answer to the question of how much we believe the eFAST result remains up in the air!
References:
- Lung ultrasound underdiagnoses clinically significant pneumothorax. EAST 2024, Podium paper 21.
- eFAST exam errors at a level 1 trauma center: A retrospective cohort study. Am J Emerg Med. 2021 Nov;49:393-398.