It’s well known that our elders do less well than younger folks after injury. The number of complications is higher, there tends to be more loss of independence during recovery, and mortality is increased. This is not only true of high energy trauma like car crashes, but also much lower energy events such as a fall from standing.
Rib fractures are common after falls in the elderly and contribute to significant morbidity if not treated adequately. Traditionally, they are identified through a combination of physical exam and chest x-ray. Unfortunately, only half of rib fractures are visible on x-ray. It falls to the physical exam to detect the rest.
A group at Beth Israel Hospital in Boston explored the utility of using chest CT in an attempt to determine if this would result in more appropriate and cost-efficient care in the elderly. They performed a retrospective study of 3 years of their own data on patients aged 65 or more presenting after a mechanical fall and receiving a rib fracture diagnosis. Imaging was ordered at the discretion of the physician. A total of 330 patients were elderly, fell, and had both chest x-ray and chest CT obtained. This was a very elderly group, with a mean age of 84 years!
Here are the factoids:
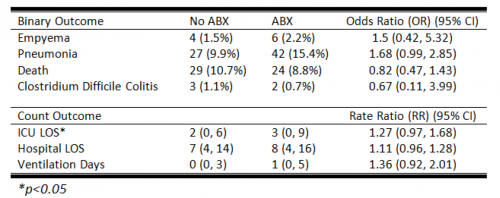
- Rib fractures were seen on chest x-ray in 40 patients (12%) and on CT in an additional 56 ; 234 patients had no fractures on either
- When fractures were seen on both studies, CT identified a median of 2 more fractures than chest x-ray
- Patients with fractures not seen on chest x-ray were admitted significantly more often than those without fractures (91% vs 78%)
- Mortality, admission to ICU, ICU length of stay, and hospital length of stay were not different if fractures were seen only on CT
- CT scan identified new issues or clarified diagnoses suggested by chest x-ray in 14 cases, including one malignancy
- Rib detail images were obtained in 13 patients and proved to be better than chest x-ray, but not quite as good as CT scan
Conclusion: use of CT for rib fracture diagnosis resulted in a few more admissions, but no change in hospital resource utilization, complications, or mortality.
Bottom line: Hmm…, read the paper closely. The authors conclude that more patients with CT-only identified rib fractures are admitted. But compared to what? Unfortunately, patients without rib fractures on CT. What about comparing to patients who had fractures seen on chest x-ray too? If that number is the same, then of what additional use is CT? Identifying a few incidentalomas?
Given that there is no change in the usual outcome measures listed here, it doesn’t seem like there is any additional benefit to adding CT. And I can see a lot of downsides: cost, radiation, and possible exposure to IV contrast. In my mind, there is still nothing that beats a good physical exam and a chest x-ray. Skip the CT scan. And don’t even think about ordering rib detail images! That’s so 1990s. And even if no rib fractures are seen on imaging, physical exam is the prime determinant for admitting your patient for aggressive pain management and pulmonary toilet.
Reference: Chest CT imaging utility for radiographically occult rib fractures in elderly fall-injured patients. J Trauma ePub ahead of print, Jan 23, 2019.