All US trauma centers verified by the American College of Surgeons are required to have clinical practice guidelines (CPG). Trauma centers around the world generally have them, but may not be required to by their designating authority. But don’t confuse a policy about clinical management, say for head injury, with a real CPG. Policies are generally broad statements about how you (are supposed to) do things, whereas a CPG is a specific set of rules you use when managing a specific patient problem.
- Look around; don’t reinvent the wheel! This is the first mistake nearly every center makes. It seems like most want to spend hours and hours combing through the literature, trying to synthesize it and come up with a CPG from scratch. Guess what? Hundreds of other centers have already done this! And many have posted theirs online for all to see and learn from. Take advantage of their generosity. Look at several. Find the one that comes closest to meeting your needs. Then “borrow” it.
- Review the newest literature. Any existing CPG should have been created using the most up to date literature at the time. But that could have been several years ago. Look for anything new (and significant) that may require a few tweaks to the existing CPG.
- Create your draft, customizing it to your hospital. Doing things exactly the same as another center doesn’t always make sense, and it may not be possible. Tweak the protocols to match your resources and local standards of care. But don’t stray too far off of what the literature tells you is right.
- Make sure it is actionable. It should not be a literature summary, or a bunch of wishy-washy statements saying you could do this or consider doing that. Your CPG should spell out exactly what to do and when. (see examples below)
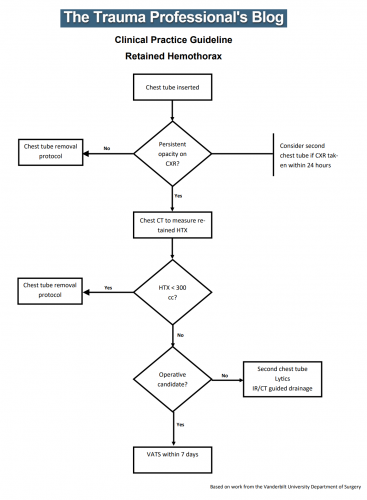
- Create a concise flow diagram. The fewer boxes the better. This needs to be easy to follow and simple to understand. It must fit on one page!
- Get buy-in from all services involved. Don’t try to implement your CPG by fiat. Use your draft as a launching pad. Let everyone who will be involved with it have their say, and be prepared to make some minor modifications to get buy-in from as many people as possible.
- Educate everybody! Start a campaign to explain the rationale and details of your CPG to everyone: physicians, nurses, techs, etc. Give educational presentations. You don’t want the eventual implementation to surprise anyone. Your colleagues don’t like surprises and will be less likely to follow along.
- Roll it out. Create processes and a timeline to roll it out. Give everyone several months to get used to it.
- Now monitor it! It makes no sense to implement something that no one follows. Create a monitoring system using your PI program. Include it in your reports or dashboards so providers can see how they are doing. And if you really want participation, let providers see how they are doing compared to their colleagues. Everyone wants to be the top dog.
Some sample CPGs: