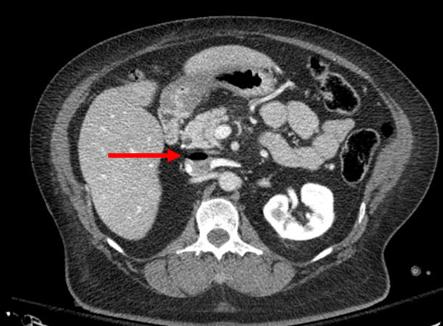
Just about every practice guideline out there regarding liver and spleen injury has some type of physical activity guidelines associated with it. The accepted dogma is that moving around too much, or climbing stairs, or lifting objects, or getting tackled while playing rugby could exacerbate the injury and lead to complications or surgery.
But is it true? Activity restrictions after solid organ injury have been around longer than I have been a trauma surgeon. And the more people I poll on what they do, the more and very different answers I get. And there are no decent papers published that look critically at this question. Until now.
A pediatric multi-center study of study on adherence to activity restrictions was published last year. Ten Level I pediatric trauma centers in the US tabulated their experience with solid organ injuries over a 3.75 year period fro 2013 to 2016. Only patients with successful nonoperative management of their injury were included, and those with high grade renal or pancreatic injuries were excluded.
Since this was a pediatric study, the American Pediatric Surgical Association (APSA) practice guideline was followed (activity restriction = organ injury grade + 2 weeks). Activity restrictions included all sports, any recreational activity with wheels, or any activity that involved having both feet off the ground. Patients with Grade III-V injuries were seen at an office visit after 2 weeks, and lower grade injuries had a phone followup.
Adherence to guidelines was assessed by a followup phone call two months after injury. Clinical outcomes assessed at 60 days included unplanned return to the emergency department (ED), re-admission, complications, and development of new bleeding confirmed by surgery, ultrasound, or computed tomography (CT) at 60 days post injury.
Here are the factoids:
- Of the 1007 patients in the study, some 56% were either excluded (178) or lost to followup (463)
- Of the remaining 366, roughly 46% had a liver injury, 44% spleen, and the remaining 10% had both
- Median age was 10, so this was actually a younger population
- 76% of patients claimed they abided by the guidelines, 14% said they did not, and 10% “didn’t know.” This means they probably did not.
- For the 279 patients who said they adhered to activity restrictions 13% returned to the ED and half were admitted to the hospital
- Of the 49 patients who admitted they did not follow the guidelines, 8% returned to the ED at some point and none were readmitted
- The most common reasons for return to ED were abdominal pain, anorexia, fatigue, dizziness, and shoulder pain
- There were no delayed operations in either of the groups
Bottom line: There were no significant differences between the compliant and noncompliant groups. Unfortunately, the authors did not include an analysis of the “I don’t know if I complied” group, which would have been interesting. But there is one issue I always worry about in these low-number-of-subjects studies that don’t show a significant difference between groups. Did they have the statistical power to show such a difference? If not, then we still don’t know the answer. And unfortunately, I’m not able to guess the numbers well enough to do the power calculation for this study.
I am still intrigued by this study! Our group originally had a fixed time period (6 weeks) of limited activity in our practice guideline for pediatric solid organ injury patients. This was rescinded last year based on our experience of no delayed complications and guidance from our sister pediatric trauma center at Children’s Hospital in Minneapolis. We are also moving toward making a similar change on our adult practice guideline.
Too many centers wait too long to make changes in their practice guidelines. They bide their time waiting for new, published research that they can lean on for their changes. Unfortunately, I think they will be waiting for a long time because many of our questions are not interesting enough for acceptance by the usual journals. Rely on the expertise and experience of your colleagues and then make those changes. Be sure to follow with your performance improvement program to make sure that they actually do work as well as you think!
Reference: Adherence to APSA activity restriction guidelines and 60-day clinical outcomes for pediatric blunt liver and splenic injuries (BLSI). J Ped Surg 54:335-339, 2019.