You are seeing a young man in the emergency department who gives a history of falling two days ago. He experienced chest pain at the time which has persisted, but he did not immediately seek medical care. He has noticed that he now gets winded when walking quickly or climbing stairs, and describes pleuritic chest pain.
He presents to your emergency room and on exam has a bruise over his left lateral chest wall. Subcutaneous emphysema is present, and breath sounds are absent. Chest x-ray shows a complete pneumothorax on the left.
You carefully prepare and insert a chest tube in the usual position. A significant rush of air occurs, which tapers off over 15 seconds. Here is the followup image:
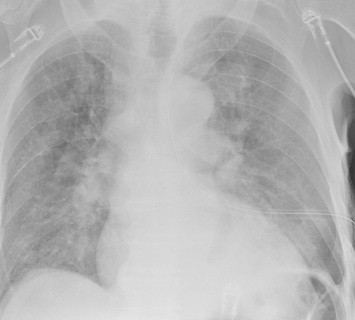
About 10 minutes later you are called to his room because he is complaining of dyspnea and his oxygen saturation has decreased to 86%. Breath sounds are somewhat decreased and the tube appears to be functioning properly. You immediately obtain another chest x-ray:

What just happened? This is a classic case of unilateral “flash” pulmonary edema after draining the chest cavity. This phenomenon was first described in 1853 in a patient who had just undergone thoracentesis. It is very uncommon, but seems to occur after rapid drainage of air or fluid from the chest cavity.
Here are some interesting factoids from case reports:
- It occurs more often in young men
- It is most common when draining large hemo- or pneumothoraces
- Rapid drainage seems to increase the incidence
- It is likely due to increased pulmonary capillary permeability from inflammatory mediators or changes in surfactant
- Symptoms typically develop within an hour after drainage
What should you do? First, if you are draining a large collection of air or blood, do it slowly. Clamp the back end of the chest tube prior to insertion (you should always do this if you value your shoes) and use it to meter the amount of fluid or air released. I typically let out about 300cc of fluid, then wait a minute and repeat until all the blood has been drained. For air, vent it for 10 seconds, then wait a minute and repeat.
In patients at high risk for this condition, apply pulse oximetry and follow for about an hour. If they still look and feel great, nothing more need be done.
References:
- Fulminant Unilateral Pulmonary Edema After Insertion of a Chest Tube. Dtsch Arztebl Int 105(50):878-881, 2008.
- Reexpansion pulmonary edema after chest drainage for pneumothorax: A case report and literature overview. Respir Med Case Rep 14:10-12, 2015.
- Re-expansion pulmonary edema following thoracentesis, Can Med Assn J 182(18):2000-2002, 2010.