Damage control surgery for trauma is over 20 years old, yet we continue to find ways to refine it and make it better. Many lives have been saved over the years, but we’ve also discovered new questions. How soon should the patient go back for definitive closure? What is the optimal closure technique? What if it still won’t close?
One other troublesome issue surfaced as well. We discovered that it is entirely possible to leave things behind. Retained foreign bodies are the bane of any surgeon, and many, many systems are in place to avoid them. However, many of these processes are not possible in emergent trauma surgery. Preop instrument counts cannot be done. Handfuls of uncounted sponges may be packed into the wound.
I was only able to find one paper describing how often things are left behind in damage control surgery (see reference below), and it was uncommon in this single center study (3 cases out of about 2500 patients). However, it can be catastrophic, causing sepsis, physical damage to adjacent organs, and the risk of performing an additional operation in a sick trauma patient.
So what can we do to reduce the risk, hopefully to zero? Here are my recommendations:
- For busy centers that do frequent laparotomy or thoracotomy for trauma and have packs open and ready, pre-count all instruments and document it
- Pre-count a set number of laparotomy pads into the packs
- Use only items that are radiopaque or have a marker embedded in them. This includes surgical towels, too!
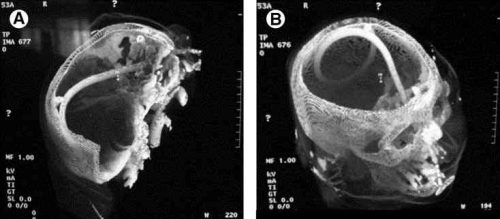
- Implement a damage control closure x-ray policy. When the patient returns to OR and the surgeons are ready to begin the final closure, obtain an x-ray of the entire area that was operated upon. This must be performed and read before the closure is complete so that any identified retained objects can be removed.
Tomorrow, a sample damage control closure x-ray.
Related post:
Reference: Retained foreign bodies after emergent trauma surgery: incidence after 2526 cavitary explorations. Am Surg 73(10):1031-1034, 2007.