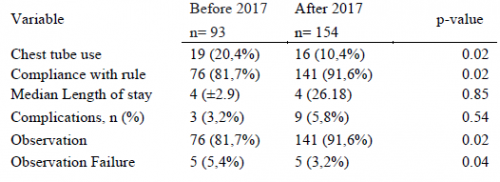
Oh, look, my favorite topic! Prevention of venous thromboembolism (VTE) and complications. We’ve grown accustomed to using enoxaparin at the standard 30mg bid dose for a long time. The orthopedic surgeons like to use 40mg qd, and there is some literature that shows this is reasonable for fracture patients.
The group at OHSU in Portland wanted to show that the single dose regimen is just as safe and effective as the bid dose. They performed a seven year, prospective, randomized trial of the two dose regimens. Weekly screening duplex exams were performed. The outcome measured was the occurrence of deep venous thrombosis (DVT) in the legs. They also examined missed doses, bleeding complications, and hospital length of stay.
Here are the factoids:
- There were 267 total patients, 139 on the single dose regimen and 128 in the bid group
- Average age was 49 and BMI was 28 in both groups
- DVT occurred in 15 (11%) qd patients and 12 (9%) bid patients
- Bleeding occurred in 19% of qd patients vs 14% of bid patients
- There were fewer missed doses in the qd patients
- None of the differences were statistically significant
The authors concluded that the qd dose was similar to the bid dose and is equally efficacious.
Bottom line: Hold on, now. First, this is a non-inferiority study. Daily dosing is presumed to be as good as twice daily dosing since there was no statistical difference seen between groups. This assumes that you have the statistical power (enough patients) to detect a difference. Is this the case here?
I pulled out my Sample Size calculator to check this over. I work things backwards to see the magnitude of difference that would have to be present for the given number of subjects. It looks like a sample size this small would only be able to detect a difference of 2x in the DVT occurrence result!
Lets look at this in simple terms. The absolute number of DVTs was actually higher in the qd group (11% vs 9%). So let’s say it is actually inferior to bid, meaning that the higher occurrence of DVT is real. Using the number of subjects here, the incidence could rise to 20% in the qd group and still not reach significance.
The other major issue is the potential for selection bias. This study took place over 7 years. Yet only 267 were enrolled, or 38 patients per year. But this trauma center admits several thousand patients annually. If the enrollment criteria were so strict, the subjects probably don’t represent the general population. And if they weren’t, where did all the patients go? This is most likely a skewed study group.
I have lots of questions for the presenter and authors on this one!
- Please show us your power calculations. Are you sure you have the statistical oomph to show non-inferiority?
- Why did it take so long to accumulate 267 subjects? Show us the statistics for your overall trauma population to make sure they look the same.
- Were you able to detect any other complications like pulmonary embolism?
Lots of questions here! Hopefully there’s much more information in the presentation!
Reference: A PROSPECTIVE RANDOMIZED TRIAL COMPARING TWO
STANDARD DOSES OF ENOXAPARIN FOR PREVENTION OF
THROMBOEMBOLISM IN TRAUMA. AAST 2021, Oral abstract 40.