Finally, a performance improvement (PI) abstract at AAST!
As many of you know, there are two general types of issues that are encountered in the usual PI processes: provider (peer) vs system. Provider issues are errors of omission or commission by an individual clinician. Examples include a surgeon making a technical error during a procedure, or prescribing the wrong drug or dose for some condition.
One might think that provider issues are the most common type of problem encountered. But they would be wrong. The vast majority of clinicians go to work each day with the idea that they will do their job to the best of their abilities. So how could things go awry?
Because the majority of errors have some degree of system component! They are set up to fail by factors outside their perception and/or control. Let’s look at a surgeon who has several small bowel anastomoses fall apart. His surgery department head chides/educates him, reports him to hospital quality, and proctors his next ten bowel cases. Everything is good, right?
But then, two months later, the stapler company issues a recall because they found a higher than usual number of anastomotic failures with one of their products. So it wasn’t the surgeon after all, like everyone assumed. This is an extreme example, but you get the idea. System issues often look like peer issues, but it’s frequently difficult for many PI programs to recognize or accept this.
A multi-institutional group reviewed the results of a newly implemented Mortality Reporting System (MRS) to analyze a large number of PI opportunities for improvement (OFI). More than 300 trauma centers submitted data to the MRS when a death occurred where an OFI was identified. The reports included details of the incident and mitigation strategies that were applied.
Here are the factoids:
- A total of 395 deaths were reviewed over a two year period
- One third of deaths were unanticipated (!!), and a third of those were failure to rescue
- Half of errors pertained to clinical management, clinical performance, and communication
- Human failures occurred in about two thirds of cases
- The most common remedy applied was education, which presumes a “provider issue”
- System strategies like automation, standardization, and fail-safe approaches were seldom used, implying that system issues were seldom recognized
- in 7%, the trauma centers could not identify a specific strategy to prevent future harm (!!!)
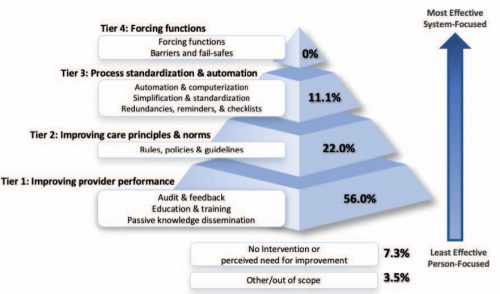
The authors concluded that most strategies to reduce errors focus on individual performance and do not recognize the value of system-level intervention.
Bottom line: Look at the pyramid chart above (interesting choice for a chart, but very effective). The arrow shows progression from provider focus to systems focus. The pyramid shows how the recognition of and intervention for system issues drops off very rapidly.
I am both shocked and fascinated by the last bullet point. A strategy couldn’t be developed to prevent the same thing from happening again. Now, there are a few rare instances where this could be correct. Your patient could have been struck by a bolt of lightning in her room, or a meteorite could have crashed through the wall. But I doubt it. This 7% illustrates the importance of investigating all the angles to try to determine how the system failed!
For once, I have no critique for an abstract. It is a straightforward descriptive study that reveals an issue that many in PI are not fully aware of. I’ll definitely be listening to this one, and I really look forward to the published paper!
Reference: ERROR REDUCTION IN TRAUMA CARE: LESSONS FROM AN ANONYMIZED, NATIONAL, MULTI-CENTER MORTALITY REPORTING SYSTEM. AAST 2021, Oral abstract #17.