Yesterday, I wrote about one of the many fractures that occurs during falls onto outstretched hands, the Galeazzi fracture. Today, I’ll describe another one, the Monteggia fracture. Yes, this one is named after another Italian surgeon! And like the other one, the person it was named after was actually the second to describe it.
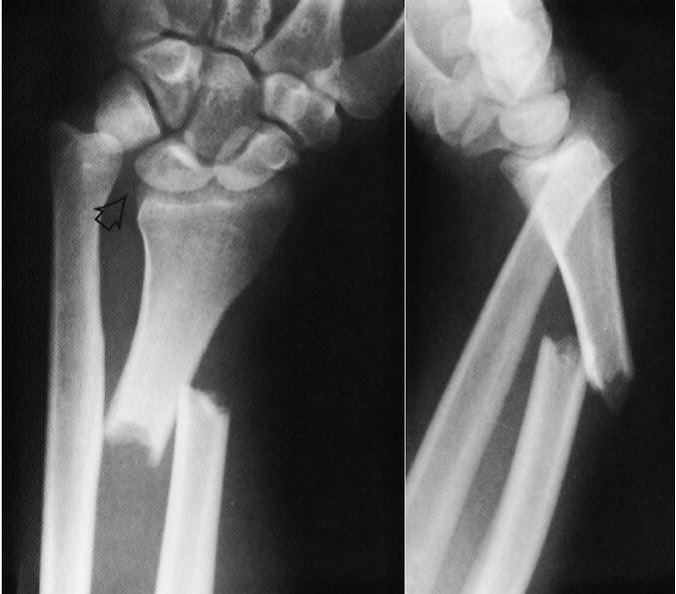
Think of the Monteggia fracture as the exact opposite of a Galeazzi fracture. The fractured bone is switched, as is the dislocation. Whereas the Galeazzi is a distal radius fracture with a distal ulnar dislocation which pulls the radio-ulnar joint apart, the Monteggia is a proximal ulnar fracture with a proximal radial head dislocation.
Here’s what it looks like:
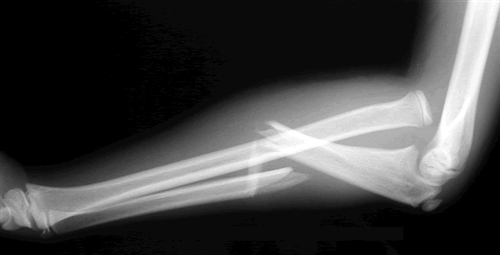
Of course, the orthopedic surgeons have a classification system for this based on the directions the bones fracture and dislocate. I won’t bore you with the details.
Unlike the Galeazzi fracture, all of these require operative repair, even in children. This helps stabilize the radial head and decreases the incidence of malunion.