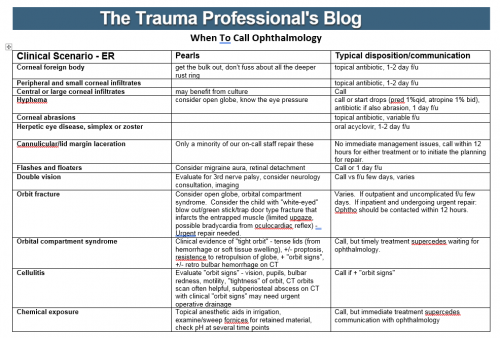
Most trauma centers have at least a few practice guidelines to help the standardize the way they manage common injuries. Solid organ injury. Elder trauma. Chest tube management. But they are all designed for use in patients who present shortly after their injury.

What about someone who presents a day or two, or more, after their injury? That changes the picture entirely. Most guidelines have a time component built in. A TBI protocol requires a repeat head CT after a certain period of time. Solid organ injury patients may have restricted activity or frequent vital signs for a while.
But all too often, trauma professionals treat the patient with delayed presentation exactly the same as fresh trauma. For example, a patient falls and bumps their head. They have a persistent headache, and after two days decide to visit their local ED. The CT scan shows a small amount of subarachnoid blood in the area of the impact. Your practice guidelines says to admit for observation, frequent neruo checks, and repeat head CT in 12 hours.
Or a young male playing sports took a hit to his left flank. After 3 days, he’s just tired of the pain and comes to the ED for some pain medication. CT scan shows a grade III spleen injury with a small amount of hemoperitoneum. Your protocol says to admit, make NPO, liimit activity, and observe for 2 days.
What would I do in these cases? Think about it! If the patients had presented right after the event, they would have gone through your guideline and would have been discharged already. So I would review the images, talk to the patients about their injuries, then send them home from the ED with followup. They’ve already passed!
Bottom line: Remember, practice guidelines are not etched in stone. Variances are possible, but need to be well thought out in advance. And hopefully documented in the chart to expedite the inevitable trauma performance improvement inquiry. If the requisite amount of time has gone by, and the history and exam are reasonable, the patient has already passed your protocol. Send them home.
Related posts: