For years, I’ve taught my residents participating in trauma activations, “Your patient is bleeding to death until proven otherwise.” This concept served as the basis of the [poorly documented] “Golden Hour” and for decades has directed our efforts at getting patients to a center with an immediately available OR as quickly as possible.
Donald Trunkey published the first paper illustrating the trimodal distribution of death in 1983 in Scientific American. A crude graph showed the large spike in early deaths that occurred within this first hour. But the paper was mainly observational and was not based on quantitative data.
Wouldn’t it be nice to know how quickly these injured patients were dying, and of what? The trauma group at the University of Pennsylvania massaged data in the state trauma database, focusing on patients who died of their injuries during the first four hours. They created two variables to more objectively compare times, the TD5 and the TD50. These are the time at which 5% and the time at which 50% (median) had died, respectively.
The Pennsylvania Trauma Outcomes Study database contains a huge amount of data. During the 11 years of the study, a total of 6,547 met the mortality criteria for analysis.
Here are the factoids:
- The mechanism of injury was about 60% blunt / 40% penetrating, with an average ISS of 33
- The majority of these patients (85%) were hypotensive before their death, meaning that they were likely bleeding to death on arrival
- The overall TD5 was 23 minutes, and the TD50 was 59 minutes
- These numbers were shorter for penetrating injuries, TD5=19 minutes and TD50=43 minutes
- Patients who were not hypotensive lived a little longer: TD5=44 minutes and TD50 = 2 hours 18 minutes
- 77% of patients died in the ED and 19% in the OR. The remainder died in the ICU.
This chart shows the TD5 by mechanism and type of surgery. This represents when after arrival, patients start dying due to their injuries. Penetrating injury plus hypotension kills the fastest at 19 minutes and head injuries the slowest at 1:20.
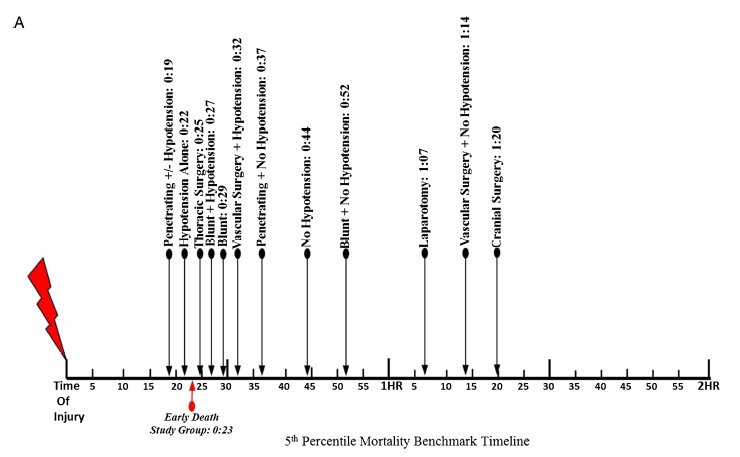
Bottom line: The authors clearly show how soon seriously injured patients start to die. It’s less than 20 minutes in victims of penetrating injury with early hypotension. And the time between the “just start do die” point (TD5) and the “half are dead” point (TD50) is frighteningly short, just an additional twenty minutes!
There appears to be a bit of a grace period in patients who arrive with a normal blood pressure. Their TD50 is extended out to about two hours. All this means is that they are bleeding more slowly, but it is still killing them.
A good rule of thumb is that ANY hypotensive patient should make you justify why you are NOT ALREADY IN THE OPERATING ROOM! Dawdling in the trauma bay or performing unnecessary scans will push your patient much closer to the point of no return. Look at the huger percentage of patients in this study who died in the ED.
Remember, your patient is bleeding to death in front of your eyes, and the only place you can stop it is the OR!
Reference: Defining the optimal time to the operating room may
salvage early trauma deaths, J Trauma 76(5):1251-1258, 2014.