A massive transfusion protocol must be available at all trauma centers, large and small, urban and rural. In an ideal setting, attempts are made to keep the ratios of red blood cells to plasma transfused somewhere between a 1:1 and 2:1 ratio. Unfortunately, many hospitals do not keep any thawed plasma because of its 5-day shelf life, so they must resort to thawing it on demand. This process is slow and may take 20-40 minutes, so it is often difficult for these centers to keep within the optimal ratios.
The group at the Guthrie Clinic, a Level II trauma center in northern Pennsylvania, tried a novel approach to thawed plasma availability: keeping two units continuously available in the ED for trauma use only. After three days, these units were returned to the blood bank for general use and were replaced with new ones. They reviewed their one year experience with wasted plasma and compared it with the two years prior to implementation.
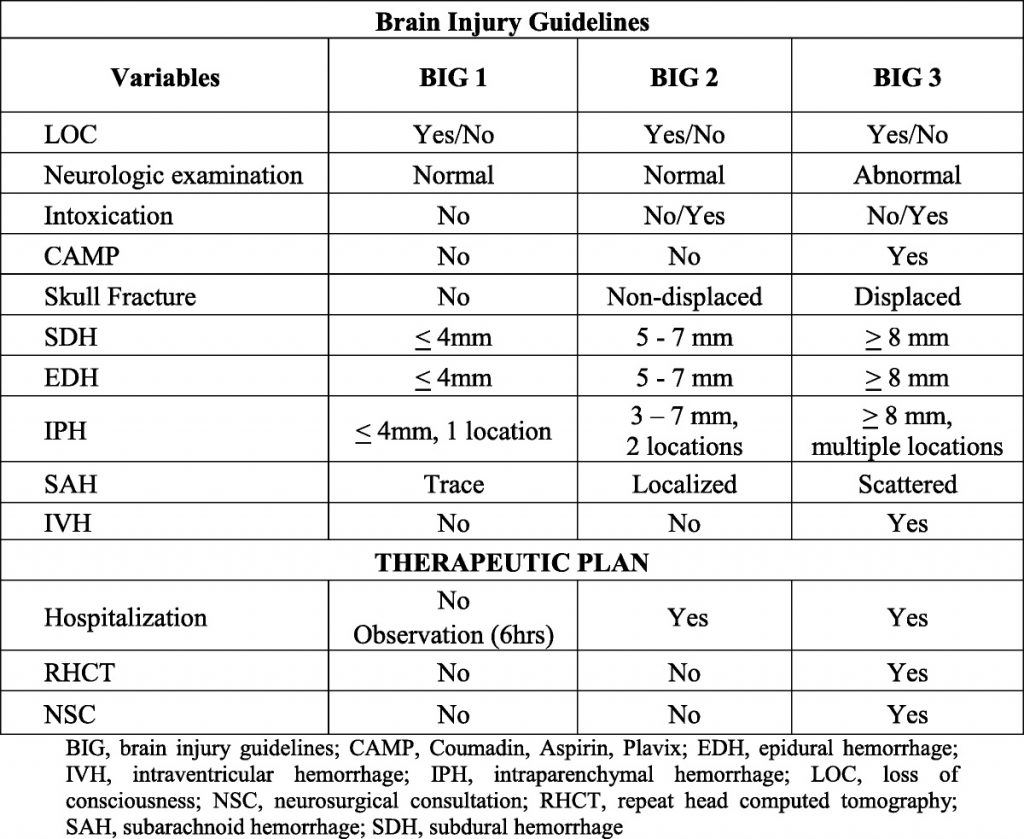
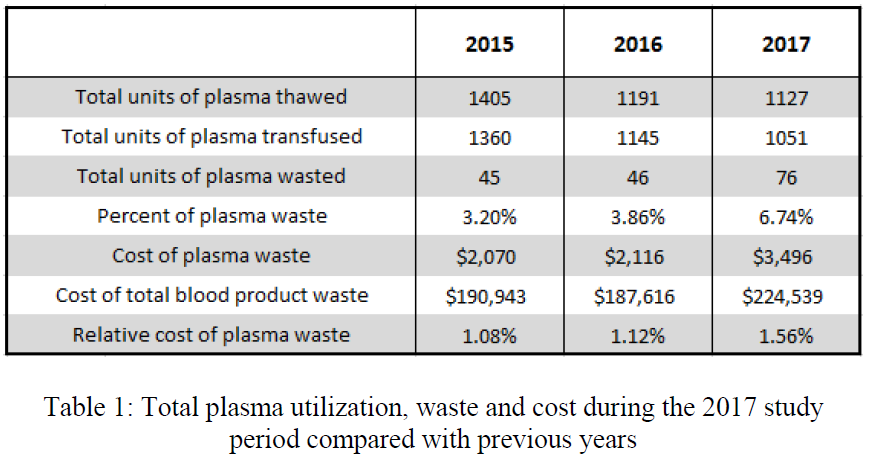
Here are the factoids:
- The blood bank thawed 1127 units during the study period; 274 units were placed in the trauma bay
- There was a significant increase in waste and cost of wasted products
- Yet the authors did not find an increase in the relative cost of plasma waste
- The average cost to maintain access to plasma in the trauma bay was $117 per month
- The authors concluded that the increased waste and cost were insignificant compared to the cost of total blood bank waste (?)
Here are some questions for the authors and presenter to consider in advance to help them prepare for audience questions:
- What do all the terms mean, like relative cost? I’m confused that the cost of waste is significantly higher, but not the relative cost. Please explain in your presentation.
- Is the $117/month to maintain access just for the refrigerator itself and any other support hardware or software? It’s not clear if this includes any part of the blood product cost.
- Why not keep the plasma in the blood bank? Even though it might still be wasted, couldn’t you save the $117 monthly and avoid the hassle of trying to find a cubby to put the ED blood refrigerator in?
- Why is 3 days your magic number? Did you consider doing a simulation after you completed the study to see what would have happened if you picked 2 or 4 days in the ED instead?
This is a very creative approach to stocking perishable goods that are infrequently used. I look forward to hearing the presentation.
Reference: A novel protocol to maintain continuous access to thawed plasma at a rural trauma center. EAST 2019, Quick Shot Paper #14.