Severe TBI consists of a primary injury to the brain, followed by swelling, vascular, and ischemic problems which may cause a secondary injury. Much of the critical care management of this injury involves avoiding or ameliorating secondary injury. This is typically accomplished via medical means first, and through surgical procedures when medical management is insufficient.
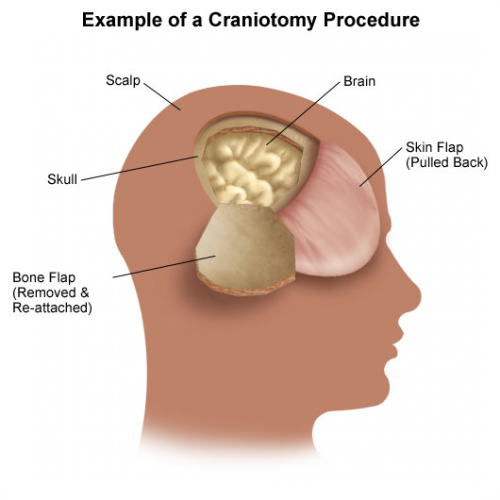
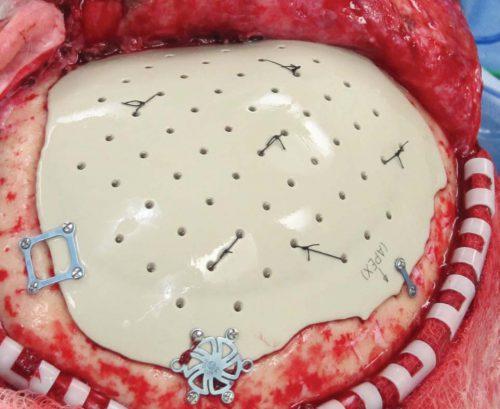
Two types of surgical decompression are currently practiced: craniotomy and evacuation of blood/clot, and decompressive craniectomy with removal of a bone flap. The latter can be performed prophylactically before severe swelling occurs, or therapeutically as a damage control procedure when ICP is refractory to all other measures.
There has been a decades-old debate as to whether craniectomy, which is a major undertaking with months of skull/bone flap management, is actually worthwhile. Most studies have examined the utility of damage control craniectomy for refractory ICP. The results have not really been convincing one way or the other.
But what about prophylactic decompressive craniectomy (DC) to avoid future ICP problems while the patient is in the ICU? The surgical group at the University of Arizona at Tucson performed a five year retrospective review of their experience. Using propensity score matching, they identified 99 severe TBI patients who underwent DC (33) or craniotomy only (CO, 66). A power analysis showed that this sample size should be sufficient to demonstrate a significant difference.
Here are the factoids:
- Both groups were similar with respect to age, GCS, ISS, AIS-head, and type of bleed
- 26% died and 63% were discharged to rehab or skilled nursing facility
- When comparing DC to CO groups, there were no differences in mortality, discharge to skilled nursing facility, discharge GCS or Glasgow Outcome Scale
- There were more complications in the DC group, including shunt insertion for hydrocephalus (9% vs 0%), and reoperation (12% vs 2%)
- Rates of wound infection and ventriculitis were the same for both groups (0-3%)
Bottom line: Although the study is small, it supposedly had enough patients for identification of significant differences. And basically, it didn’t show a positive difference for prophylactic decompressive craniectomy. There is certainly some opportunity for selection bias by the neurosurgeons that cannot be controlled for by this retrospective design. But it is yet another piece of the decompressive craniectomy puzzle.
Overall, the literature support for either prophylactic or damage control craniectomy is not very strong. If it were, we would have identified some real benefits by now. What we don’t know is if there are specific subgroups of severe TBI patients who might benefit from it. So if your center is not involved in a project to study this, you should probably ask your neurosurgeons to base their practice only on what we know about this procedure to date.