Delayed Splenic Rupture: Part 2
Yesterday I wrote about the history of “delayed splenic rupture.” Today I’ll discuss how to deal with it.
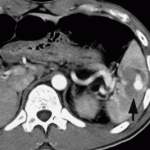
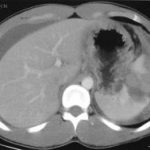
If possible, try to avoid ever having to mess around with this clinical problem. If you order an abdominal CT after blunt trauma and see a splenic contrast blush of either type (pseudoaneurysm or extravasation, see left photo), then deal with it before the patient even knows he has a problem. A trip to interventional radiology will usually solve the problem. And if embolized, these patients almost never come back with a bleeding problem.
As I’ve said many times before, if the patient is hemodynamically compromised, then an OR visit is required. The usual solution is splenectomy. Some recommend repairing the spleen, but this is technically more difficult than it sounds, and it is difficult for the surgeon to sleep soundly after performing one of these.
Lets say you inherited one of these from someone else, or ignored the warning signs on the initial CT. The usual time frame for presentation to the ED with acute bleeding is 7 to 10 days after the initial injury. If they are not stable, physical exam or FAST will quickly direct you to the OR, once again for splenectomy. Some patients will stabilize with fluids and can safely be sent to CT scan.
Once the CT confirms what the problem is, a trip to interventional radiology is in order if the patient remains stable. Here is the key: the radiologist must embolize something! If they find a bleeding vessel, then they can selectively embolize it. If they don’t, then the main splenic artery should be embolized. This will decrease the arterial pressure head, but won’t eliminate it. It will decrease the likelihood of additional bleeding as much as possible.
At this point, the patient should be admitted to the trauma service and monitored using your solid organ injury protocol. If they have any hemodynamic issues, it’s time to remove the spleen. Remember, this is the third time they’ve had a problem, and like in baseball, their spleen is out! Attempted splenorrhaphy at this point is pointless and may lead to yet another operation.
Related posts: