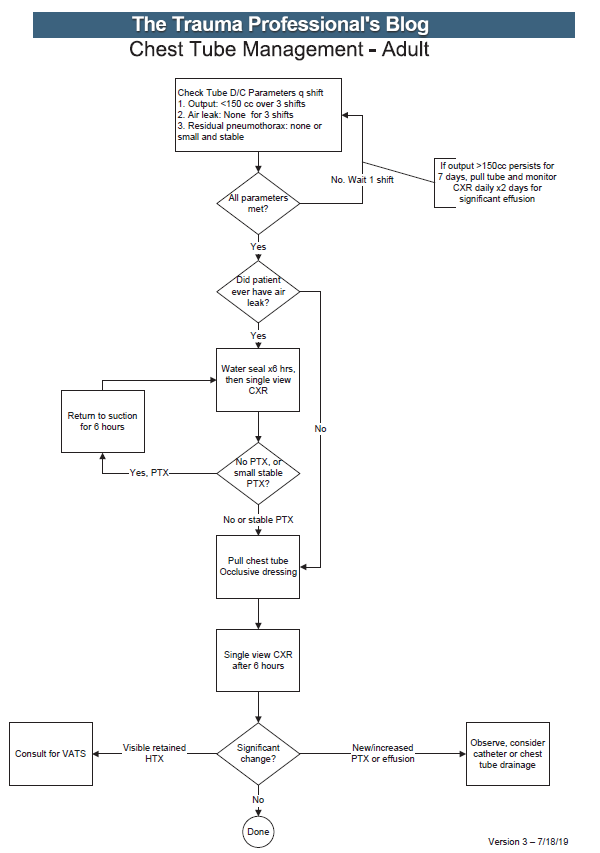
Osteoporosis is a major risk factor for fractures in our elderly patients, particularly postmenopausal females. A number of treatment modalities have been introduced, primarily medications. One of the less expensive of these is salmon calcitonin nasal spray. This drug has been used sporadically in the spine surgery community and I’ve always been curious about the supporting data. So I finally looked into it and wanted to share my findings.
The earliest research that most tend to hang their hat on is the Prevent Recurrence of Osteoporotic Fractures (PROOF), a five-year study from the early 90’s published in 2000. It was a double-blind placebo controlled study involving 47 centers in the US and UK. All subjects received supplemental calcium and Vitamin D daily. They also received one of three different doses of nasal calcitonin or a placebo daily.
Completion rates were dreadful. A total of 1,255 women with established osteoporosis started the study. Only 783 made it to 3 years and 511 to 5 years. Partly, this was due to the fact that the patients’ repeat bone density results were unblinded to the investigators, and the “statistically significant” increase in this value was only about 1.5%. I don’t believe that this is even close to clinically significant. Thus, the investigators and patients may have seen this as ineffective, dropped out of the study, and switched to some newer treatment.
As I mentioned, the study used a placebo dose of nasal spray, as well as 100, 200, or 400 unit doses daily. It is puzzling that only the 200 unit dose showed any statistically significant decline in recurrent spine fracture rate. Since the numbers in each analysis group were low due to patient attrition (about 275 in each group), this raises the question of whether this is a statistical anomaly. One would expect to see some sort of dose-response curve, not just a result only for the middle of the road dose.
The authors concluded that the 200 unit dose significantly reduces the risk of new vertebral fractures in postmenopausal women with osteoporosis. And unfortunately, multiple authors and clinicians have taken this to heart without questioning the real results in the paper.
A newer study, Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL), published in 2012, compared oral vs nasal calcitonin in 565 women. Their endpoints were changes in bone density and markers of bone turnover. The oral group had a whopping increase (haha) in lumbar bone density of 1.5%, while nasal and placebo dosing were the same at about 0.5-0.8%. This is in no way clinically significant. And paradoxically this bone density increase appeared to peak by week 24 and then slowly decline.
Similarly, bone turnover markers decreased. Interestingly, of the three markers measured, some were statistically significant for oral, nasal, and even placebo. There was no consistent pattern. And furthermore, there is no way to translate these lab tests into risk for fracture.
Bottom line: Although nasal calcitonin spray is cheap (about $1 per day), there is no valid literature that supports that it decreases the risk of future vertebral fractures. Always remember that there is a difference between what is significant to the statisticians, and what is clinically significant to your patient. Incidentally, between 10% and 40% of these patients had adverse effects from the medication, usually GI in nature. So if a drug has a measurable risk but little actual benefit, is it worth even a buck?
Osteoporosis takes decades to develop and become clinically significant. It is unlikely that a quick fix like a drug, sprayed, swallowed, or injected will make a difference in the short term. The best treatments are free (diet and exercise) but must be adopted long before it becomes a problem.
References:
- A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. Am J Med 109(4):267-276, 2000.
- A phase 3 trial of the efficacy and safety of oral recombinant calcitonin: The oral calcitonin in postmenopausal osteoporosis (ORACAL) trial. J Bone Mineral Res 27(8):1821-1829, 2012.