Happy Thanksgiving!
Monthly Archives: November 2018
GCS At 40: The Current GCS Scale (Adult)
My last post provided some history about the original Glasgow Coma Scale (GCS). Today, I’ll provide some of the finer details of measuring the components of the current iteration of GCS (not GCS-40). I will list out the individual scale values, and explain some of the most misunderstood.
As you know, there are three components to the GCS. Let’s examine each:
Eye Opening
- 4 – This is an easy one. The eyes are open, and they are opened spontaneously.
- 3 – Eyes open to your voice. If your patient is asleep and they awaken, the E score is actually 4. If they only open their eyes to repeated voice prompts, then it is a 3.
- 2 – Eyes open only to pain or stimulation. This is typically tested by squeezing a fingernail, but the exam should progress as described in the Nuances section below.
- 1 – This one is easy, too. The eyes don’t open, no matter what.
What if the eyes are swollen shut? Then record it as E1c (c = closed).
Verbal Response
- 5 – Your patient is oriented and converses with you spontaneously.
- 4 – Confused. This means that you can talk with your patient and they respond in sentences, but you can detect some confusion or disorientation based on their speech.
- 3 – Inappropriate words. Remember it this way: your patient speaks like a 3-year-old. They can say a few words but can’t construct a meaningful sentence.
- 2 – Incomprehensible sounds. This means that your patient may moan or make noises, but does not form any words.
- 1 – No verbal response at all.
If the airway is controlled with an endotracheal tube, then the score is recorded as V1t.
Motor response
- 6 – Your patient obeys commands.
- 5 – Localizes to pain. Your patient will move toward a painful stimulus in an attempt to remove it. They can move their arms/hands above their chin in response to facial stimulation.
- 4 – Withdrawal from pain. Patients cannot move their arms above the chin.
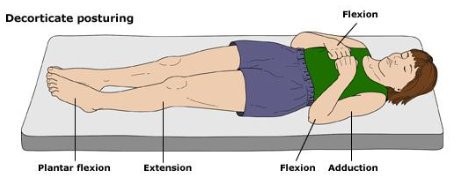
- 3 – Flexor response (decorticate posturing). This score, and the next one (2), are the ones that I always confuse. Just remember that the patients reach for the “core.” They flex their forearm and wrist, clench their fist, extend their legs, and point their toes (plantar flex).
- 2 – Extensor response (decerebrate posturing). These patients bring their arms to their sides (adduct), extend the elbow but flex the wrist and fingers, and pronate the forearms. Legs and feet are the same as above.
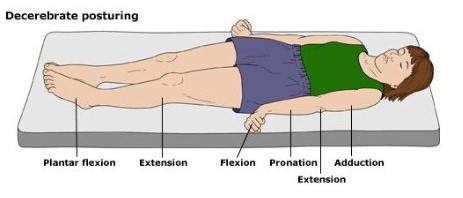
- 1 – No response to stimuli.
Nuances
- Record the entire score. This means all components and modifiers. An example would be E3 V4 M4 = 11, or E1c V1t M3 = 5, or E1 V1 M2rt M3lt = 4/5
- Alcohol or drug intoxication will interfere with accurate measurement of the GCS, especially with the verbal and eye-opening components.
- If the motor score is asymmetric (higher on one side than the other), record the higher score. Or better yet, break out the motor scores for both sides so your friendly, neighborhood neurosurgeon has a better idea of what is going on.
- Stimulation should proceed from fingernail squeeze, to pinching the trapezius muscle, to pressure in the supra-orbital notch, in that order. The sternal rub is to be discouraged, as it can lead to bruising.
In my next post, I’ll describe the differences in the Pediatric Glasgow Coma Scale.
GCS At 40: The Original GCS
The Glasgow Coma Score (GCS) has been in use for more than 40 years. Since that 40th anniversary a few years back, there has been talk of updating this tried and true system. But where did this scale come from? How was it devised? And why are we looking to update it now? I’ll dig into this topic over my next several posts.
The original paper describing the GCS was published in 1974 by Graham Teasdale and Bryan Jennett. They were neurosurgeons at the Institute of Neurologic Sciences in Glasgow, Scotland (of course) and were based in the Southern General Hospital. Until this paper was published, each report in the literature described its own assessment of level of consciousness. Most divided the spectrum into various steps noted between fully alert and comatose. Unfortunately, these systems were confusing, and they varied from 3-17 steps! There was just no consensus. Some relied on a comprehensive neurologic exam, including brainstem function tests. However, none of these were really designed for repeated bedside assessment.
Teasdale and Jennett settled on three simple areas to examine: eye-opening, motor response, and verbal response. They selected easily observable responses for each of these components. Here is a copy of the original scale:
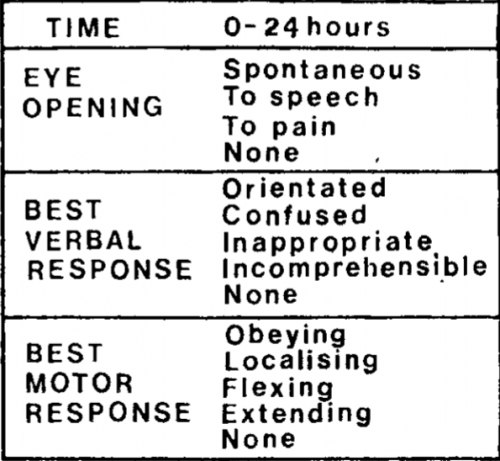
Notice that this differs from the current-day score. The motor response did not have a “withdrawal” option, so the maximum score was only 14! But that didn’t matter much at the time; the individual components were graphed out over time for inspection. A total score was not generally calculated.
Teasdale and Jennett found that inter-rater reliability for this system was excellent, compared to a 25% discrepancy for other less objective systems in use at the time. This led to its rapid adoption over the coming years.
In my next post, I’ll describe how GCS came to be used over the ensuing years.
Radiation Exposure From Imaging At Adult vs Pediatric Trauma Centers
Anyone who reads this blog already knows I am a big believer in well-crafted and focused practice guidelines. And by focused I mean directed toward a clinical problem that typically sees a lot of variability between care providers. Use of imaging is one of these clinical problems. A surgeon may order a certain set of studies for a major blunt trauma patient, and their emergency medicine colleague might order a somewhat different set for someone with the exact same history, physical exam, and injury pattern. Who is right? Neither!
And the variability is even greater when we throw a pediatric patient into the mix. Trauma professionals tend to be even more “generous” when ordering studies on children because they are afraid they might miss something. Unfortunately, this has the potential for overuse of imaging and exposure to unnecessary radiation.
Avery Nathens and a consortium of pediatric trauma centers used the Trauma Quality Improvement Database (TQIP) to review CT imaging practices on children age < 18 over a four year period. Only blunt trauma patients were studied, and the Abbreviated Injury Scale had to be at least 2 for a minimum of one organ system. Transfer patients were excluded because there is no data on imaging for the referring hospital in the TQIP database for them. Comparisons were made between practices at adult trauma centers treating children (ATC), mixed adult/pediatric centers (MTC) and pediatric only trauma centers (PCT).
Here are the factoids:
- Over 59,000 pediatric trauma patients were identified in the data, and about half (31,081) received at least one CT scan
- The distribution among the three types of trauma centers was even, with roughly a third seen at each
- Of the study group 46% had a head CT, 17% a chest CT, and 26% underwent abdominal CT
- Injured children were more likely to undergo CT if they were older, had a higher ISS, lower motor GCS, were involved in a car crash, or had severe injuries to head or torso
- Overall CT rates were about the same across the three types of centers (56% ATC, 57% MTC, 43% PTC)
- Chest CT was performed 8x as much at ATC/MTC vs PTC (!)
- Abdominal CT was performed 2x as much at ATC/MTC vs PTC
- Lesser injured children received relatively more CT scans at ATC/MTC when compared to PTC
- Using standard estimates of cancer risk from all CT scans received, children treated at adult or mixed trauma centers received enough radiation to cause 17 additional lifetime cancers per 100,000 patients
- About 35 additional lifetime cancers per 100,000 would be caused by the chest and abdominal scans performed at the ATC/MTC centers when compared to pediatric-only centers
Bottom line: This is yet another reason to adopt a well-designed pediatric imaging guideline. Not only are adult centers using CT scanning much more that pediatric-only centers, but they are unnecessarily adding to the lifetime risk for cancer of our children!
As I always recommend, find a well-designed imaging guideline from an established pediatric center and “borrow” it. Sure, it may need a few minor tweaks to fit well with your hospital. That’s okay. Just get it done so your team can begin to order the initial imaging studies consistently and intelligently.
Reference: Computed tomography rates and estimated radiation-associated cancer risk among injured children treated at different trauma center types. Injury 2018, in press. https://doi.org/10.1016/j.injury.2018.09.036
Bullet In The Disk Space: Big Deal Or Not?
In an earlier post, I reviewed the problems with lead poisoning that can occur if a bullet remains in contact with a joint space / synovial fluid, or ends up in the GI tract. But what about if it comes to rest in an intervertebral joint space? They’re dry, right?
The first case report I could find dates back to 1981. A male presented to Parkland Memorial Hospital 12 years after a gunshot to the abdomen in which the bullet lodged in a disk space. He was treated for a GI bleed, but was also noted to have many signs and symptoms of high lead levels. These included irritability, anemia, headache, lethargy, muscle weakness and confusion. A blue line was noted on the gums. X-ray of the lumbar spine showed the bullet fragment in the center of the disk space, and a cystic mass in the prevertebral area that appeared radiodense as well. Blood lead levels were elevated. The patient underwent diskectomy, resection of the mass, chelation therapy, and recovered.
Another case report from 2010 was similar in many ways. The patient was young, had a gunshot 5 years previously, and presented with symptoms of lead poisoning. The appearance of the bullet in the disk space was similar to the last case, in that the bullet could be seen within it, and there appeared to be additional radiopaque material surrounding it. It almost looked like lead was flowing out of the bullet into the disk. This case was also treated with surgical removal and chelation with a successful result.
A literature review was conducted 15 years ago that examined other case reports of bullets in the spine. Over a 25-year period 238 patients were identified with this injury. Only 12 had bullets or fragments in the disk space. All were tested for plumbism, and only one was positive. He underwent diskectomy and resection with resolution of the high lead levels.
Bottom line: We know that a bullet in contact with synovial fluid is bad, with rapid leaching of lead into the circulation. There are also suggestions that lead in contact with CSF can cause a similar problem. However, the intervertebral disk space is usually considered to be “dry” and doesn’t usually cause a problem.
However, patients with a bullet in this location should be cautioned that they do have a small risk of developing lead poisoning. They should be tested about six months post-injury to see if lead levels are on the rise. They should also be cautioned to report the development of new back pain. Structural disruption by the bullet may slowly lead to anatomic changes that result in chronic pain. And be very suspicious if there is radiopaque material in the disk space in addition to the bullet itself!
References:
- Acute lead intoxication from a bullet in the intervertebral disk space. JBJS 63A(7):1180-1182, 1981.
- Lead Poisoning by Intradiscal Firearm Bullet. Spine 35(4):E140-E143, 2010.
- Long-Term Clinical Manifestations of Retained Bullet
Fragments Within the Intervertebral Disk Space. J Spinal Disord Tech 17(2):108-111, 2004.

