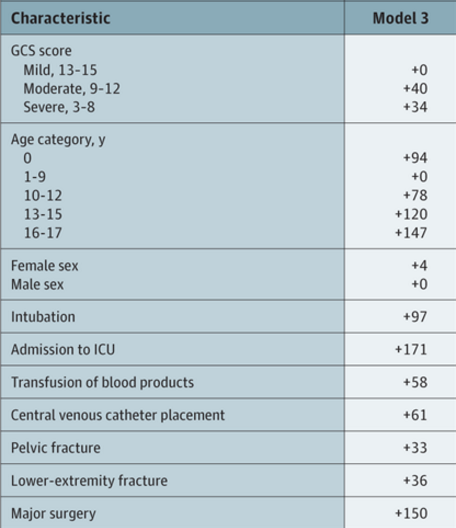
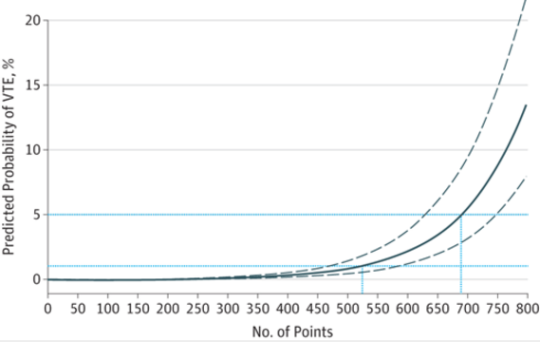
In my last post, I reviewed a study that looked at monitoring factor anti-Xa for the purpose of just “hitting the number.” Not very convincing. Today, I’ll review one that studied a reasonable outcome, the actual occurrence of VTE in patients.
This was another small, prospective study at a busy Level I trauma center. The outcomes that were analyzed included LOS, transfusion requirement, hematocrit on discharge, and diagnosis of deep venous thrombosis (DVT) or pulmonary embolism (PE). Only the last two of these make sense, especially for this small study. (205 patients in two 10 month periods).
At this center, all trauma patients are started on enoxaparin, regardless of injury severity. And all patients have sequential compression devices applied unless contraindicated by their injuries. Patients were included if the were administered 3 consecutive enoxaparin doses and had a trough anti-Xa level measured an hour before the fourth dose. If the trough was less than 0.1 IU/ml, dosing was adjusted until it rose to > 0.2 IU/ml. Outcomes were compared to historical controls from the prior year.
Here are the factoids:
- A total of 87 study patients were enrolled in 10 months. However, this represents only about 15% of trauma admissions to the center. Why were so few eligible for inclusion?
- 84% of study patients did not “hit the number” with 30mg bid dosing (again!)
- They were compared to 118 control patients who received enoxaparin during the same 10 month period, a year earlier
- Screening by duplex ultrasound was only done for “clinical suspicion” of DVT or PE. No routine screening. And we know how reliable clinical suspicion can be.
- 84% of patients were not at their anti-Xa goal when the first trough was done. Most of these patients needed 40mg bid to “hit the number.”
- DVT and PE occurrences were “significantly lower” in the dose adjusted group compared to historical controls (1.1% vs 7.6%). Now this is a difference between only 1 adjusted patient and 9 controls, and the p value barely made it at 0.046.
- Proximal DVT occurred in no adjusted patients vs 2 controls (not significant)
- PE occurred in no adjusted patients and 1 control (not significant)
- Distal DVT occurred in 1 adjusted patient and 6 controls (not significant
Bottom line: This is yet another (very) small study. It also demonstrates why you must read the study, not just the abstract! The study group was a fraction of all of the patient admitted, even though all patients supposedly received prophylaxis. The attending physicians decided when to start dosing, and this varied from 0 to 4 days. Screening was ordered only if there was some kind of clinical suspicion for DVT or PE, and the details were not spelled out.
For all these reasons, there are many, many opportunities for bias. But probably the most important problem is the statistics. I always worry when the p value for a numerical difference barely reaches 0.05, especially when the actual numbers look to be far apart. It is usually an indicator of small study size.
But in this case, the breakdown of VTE location is critical. The sums of the distal, proximal, and pulmonary occurrences show a p value difference just under 0.05. But when you compare study vs control for each, the bulk of the numbers are due to distal DVT. The literature does not convincingly support prophylaxis for distal DVT, and we do not even treat it at my center. We continue surveillance to make sure it doesn’t creep up into the popliteal arteries.
This is yet another weak study trying to make the case for anti-Xa monitoring that doesn’t pass muster. Again, we see that 30mg bid doesn’t “hit the number” without adjustment. But we also haven’t shown that hitting that magic number of 0.2 IU/ml (peak or trough) by adjusting the dose makes a difference either.
But we continue to try. In my next post, we’ll look at another recently published study on the same topic.
Related posts:
- Enoxaparin And anti-Xa Levels: Who Cares? Part 1
- Enoxaparin And anti-Xa Levels: Who Cares? Part 1.5
- Enoxaparin And anti-Xa Levels: Who Cares? Part 2
- Enoxaparin And anti-Xa Levels: Who Cares? Part 3
- How long are trauma patients at risk for VTE?
- How long does VTE risk last in TBI patients?
Reference: Association between enoxaparin dosage adjusted by anti-factor Xa trough level and clinically evident venous thromboembolism after trauma. Jama Surg. Published online ahead of print July 6, 2016.