Coagulopathy is the bane of every trauma professional. Trauma patients are bleeding to death until proven otherwise, and once they start bleeding, it only gets worse. A key component of this issue is the presence of fibrinolysis, which commonly occurs after severe trauma. Although the prime objective in managing these patients is definitive control of bleeding, antifibrinolytic therapy such as tranexamic acid (TXA) may be beneficial during the time before that can happen.
The trauma group at Denver Health has been studying fibrinolysis and finding things to do with TEG machines for many years. They postulated that, since hemorrhagic shock can cause hyperfibrinolysis (HF) and early administration of agents like TXA seem to work better when given early, wouldn’t it be nice to have a more objective way of identifying it as early as possible?
They designed a prehospital study that used end-tidal CO2 monitoring in the ambulance and correlated results with a TEG reading upon arrival at the hospital. The study was prospective and observational and involved two Level I trauma centers. End-tidal CO2 was measured from the ventilator circuit or via nasal cannula capnography. The authors compared this reading to other possible shock indicators, such as systolic blood pressure and the shock index.
Here are the factoids:
- A total of 138 patients were studied, and 13 had hyperfibrinolysis identified on hospital arrival
- Of the 13, 9 required massive transfusion, and eight died
- An ETCO2 value <17 mm Hg was determined to have a positive predictive value of 27% and a negative predictive value of 95%
- The area under the receiver operating characteristic curve was 0.71, which was better than the blood pressure (0.58) and shock index (0.54)
The authors concluded that the ETCO2 was an accurate, objective, inexpensive, and noninvasive method of measuring the risk of hyperfibrinolysis that could guide the use of agents such as TXA.
Bottom line: A lot is going on in this abstract. The central concept is that it is trying to identify a surrogate for TEG-identified fibrinolysis available in the field. It compares ETCO2 with two other semi-objective indicators, blood pressure and shock index (pulse divided by blood pressure).
The biggest issue is that the number of patients with fibrinolysis was very small, only 13. Statistical comparisons of variables between the two groups are difficult because the number of HF patients in several subgroups was only 4 or 5.
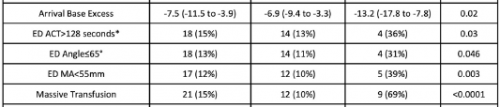
The sensitivity, specificity, and positive/negative predictive values are so-so. If the ETCO2 is above the 17mm Hg threshold, the likelihood of patients not having HF is good at 95%. But if it is below, the likelihood that they actually have HF is only 27% (true positive rate).

The area under the curve calculations is also not very impressive. Yes, an AUC of 0.71 is better than 0.54-0.58, but it is still not great.
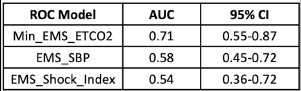
One always has to be careful finding surrogates (ETCO2) for things you really want to measure (TEG LY30 > 3%). Many potential confounders can limit their usefulness. And this case is no different, which should be apparent from the numbers. Perhaps the data would be better if a much larger group of patients were studied. Unfortunately, this will probably take close to 1,000 subjects and require a multicenter trial.
This is interesting preliminary work. It’s definitely not enough to change practice now. But with more work, and more patients, who knows?
Reference: Prehospital ETCO2 predicts hyperfibrinolysis in injured patients: implications for early use of antifibrinolytics in trauma. EAST 2024 Podium paper 3.

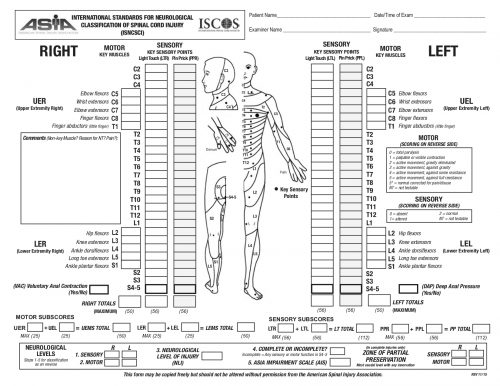
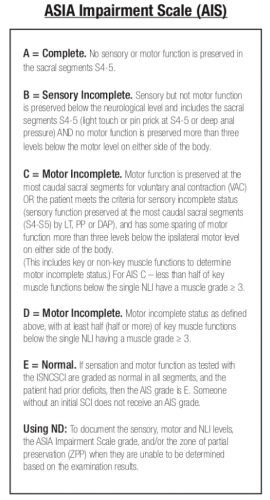 The AIS is not very granular, meaning that each step in the scale represents a large difference in function. Could patients have had improvements that did not change the AIS score but were functionally significant for the patient? For example, an improvement from a C5 to a C6 level makes a big difference in daily activities.
The AIS is not very granular, meaning that each step in the scale represents a large difference in function. Could patients have had improvements that did not change the AIS score but were functionally significant for the patient? For example, an improvement from a C5 to a C6 level makes a big difference in daily activities.