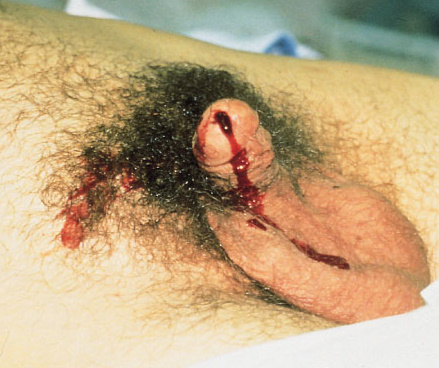
You’re seeing a trauma patient, probably a transfer from somewhere else. Either they told you there “may have been” some blood at the tip of the urethra, or maybe you see it smearing the outside of a urinary catheter that’s already in place! How do you proceed from here?
First, try not to get into that situation. Make sure that everyone on your team knows that gross blood at the meatus, male or female, means urethral injury until proven otherwise. If it’s not gross blood, it could be that the patient was incontinent and has hematuria from other causes. The fear with passing a catheter across a urethral injury is that it may convert a partial tear to a complete one. Reconstruction and complications from the latter are far more serious.
But the catheter is there. What to do?
First, leave the catheter in place. You must assume that the injury is present, and you need to rule it in or rule it out in order to decide what to do with the catheter. If the injury is not really there, then you can remove the catheter when indicated. If it really is present, then the urethral injury is being treated appropriately.
Next, do a urethrogram. I’ve previously described how to do it here, but the technique I describe is only appropriate for uncatheterized patients. The technique must be modified to use thin contrast and a method to inject alongside the catheter. To do this, fill a 20-30cc syringe with contrast (Ultravist or similar liquid) and put an 18 gauge IV catheter on the tip (no needles, please). Slide the IV catheter alongside the urinary catheter, clamp the meatus with your fingers, pull the penis to the side and inject under fluoroscopy. The contrast column will not be as vivid as with a regular urethrogram because it is outlining the urinary catheter, so there is less volume.
If the contrast travels the length of the urethra and enters the bladder without leaking out into soft tissue, there is no injury. If there is contrast leakage, stop injecting and plan to call a urologist.
Finally, be on the lookout for associated injuries. Urethral injuries are frequently found in patients with anterior pelvic fractures and perineal injuries.
Related post: