Extraperitoneal bladder rupture is a relatively uncommon injury, but is easily managed in most cases. It is associated with a blunt mechanism, and concomitant fracture of the pubic rami or spreading of the symphysis pubis is nearly always present. In the old days, we used to think that the bladder injury was due to penetration anteriorly by bony fragments, but this is probably an old wives tale. It’s more likely due to hydraulic forces occurring within the bladder at the same time the pelvic ring is being deformed or spread apart by blunt forces.
If you obtain a pelvic x-ray during the initial trauma evaluation and see any fractures or diastasis around the symphysis, think bladder injury. Placement of a urinary catheter will typically drain plenty of urine, which will usually be grossly bloody.
Once the injury is suspected, the diagnostic test of choice is a CT cystogram. Don’t confuse this with the images seen when the bladder passively fills with contrast when the catheter is clamped. There is not enough pressure in the bladder to guarantee that contrast will leak out, so this type of study may be falsely negative.
True CT cystogram technique requires filling the bladder with at least 350cc of dilute contrast under pressure by hanging it on an IV pole, then clamping the catheter. Once the bladder is filled, the scan can proceed as usual. But after it is complete, a second limited scan through the pelvis must be performed after the contrast has been evacuated by unclamping the catheter. This allows visualization of small contrast leaks that might otherwise be masked by all the contrast in the bladder.
Here’s a nice sagittal image of an extraperitoneal injury from radiologypics.com:
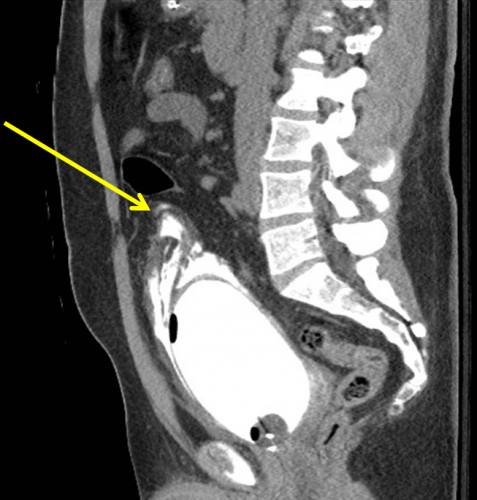
Note how the contrast dissects around the bladder but does not enter the peritoneal cavity.
Extraperitoneal injuries usually do not require repair and will heal on their own. However, if the symphysis pubis needs instrumentation to restore anatomic position, concomitant repair of the bladder is frequently necessary to keep the hardware from being contaminated by urine.
Bottom line:
- Suspect an extraperitoneal bladder injury in anyone with bony injuries involving the symphysis pubis.
- Don’t order a urinalysis in trauma patients!
- Use CT cystogram technique to make the diagnosis.
- Treatment is simple: leave the urinary catheter in place for 10 days. No urology consult is needed.
- Then repeat the CT cystogram to confirm healing, and remove the catheter.
Related posts: