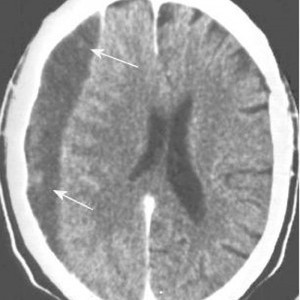
Blunt cerebrovascular injury (BCVI) is one of those low incidence, high consequence injuries like traumatic aortic rupture. Unfortunately, BCVI has gotten less attention and is less frequently screened for during trauma evaluation. There are two choices of screening criteria: digital subtraction angiography (DSA) or CT angiography (CTA). Previous work by the UT Memphis team showed that the sensitivity of evaluation by 32-slice CT angio is only 51%.
Although DSA has been considered the gold standard, there are several drawbacks. First, it is invasive and has its own collection of complications, including stroke. And it is not available at all centers, and even in those that have it, it is frequently a limited resource. Current 32-slice CTA risks missing significant injuries, which can also lead to stroke.
The UT Memphis team has now followed up their 32-slice work with a new study evaluating 64 slice scanners. Here are the factoids:
- Over a one year period, 594 patients underwent both CTA and DSA for evaluation of BCVI
- 128 patients had 163 injured vessels (!), 61% carotid and 39% vertebral
- 5 DSA complications occurred (1%): 3 puncture site hematomas and 2 iatrogenic dissections
- 64-slice CTA had an improved sensitivity of 68%
Bottom line: CT angiography for blunt cerebrovascular injury is following the same trajectory that it did for evaluating traumatic aortic injury. As the resolution of the scans improves, our diagnostic accuracy does the same. CTA using a 64-slice scanner will now begin to displace digital subtraction angiography due to its readier availability and low complication rate.
BUT, the most important part of the process is that the trauma professional needs to use solid criteria to make the decision to use it!
Related posts:
Reference: Blunt cerebrovascular injury screening with 64-channel multidetector computed tomography: more slices finally cut it. AAST 2013, Paper 13.