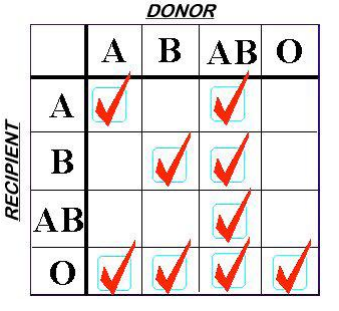
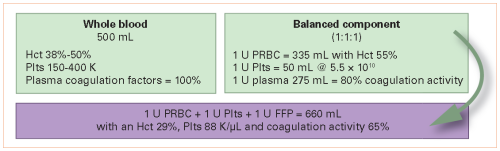
What goes around comes around. Fifty years ago, blood banks began fractionating whole blood into separate products so that specific component therapy could be administered. Over a relatively brief period of time, the switch to components became nearly complete, and whole blood was not available for civilian use. For decades, trauma professionals have had to treat our trauma patients losing whole blood, but having only components available to replace it. Unfortunately, taking blood apart and putting it back together just isn’t the same, as you can see below:
In recent years, there has been a significant movement to reintroduce whole blood. Many trauma centers are experimenting with it, and it seems we are having to relearn how to use it again. At last year’s EAST meeting, the US Army Institute of Surgical Research presented a paper that demonstrated improved survival in select severely injured patients. This year, an abstract from the University of Texas in Houston is being presented that explores the safety of giving whole blood.
This was a single-hospital study where cold-stored low titer type O whole blood (WB) was stocked in the center’s helicopters and emergency department. Components were also available. The center reviewed their 7 month experience with trauma patients who received either type of product. Their outcome variables were safety profile and transfusion reaction rates.
- 161 patients received component therapy and 95 received WB during the study period
- ISS was statistically similar, but the abbreviated injury score for chest was higher in the whole blood group (see first two bullet points below)
- Whole blood patients were more markedly impaired in the prehospital setting (higher pulse and lactate, lower blood pressure)
- Whole blood patients received fewer units of products after leaving the ED, which is an 80% reduction when matched for the usual variables (0 vs 3)
- Mortality was the same in the two groups (26% WB vs 22% component)
- There was only one transfusion reaction, and it occurred in the component group
The authors concluded that whole blood appeared to be a safe alternative to 1:1 component therapy, and was associated with a reduced need for post-ED transfusion.
- How were patients selected to receive components vs whole blood? Or were they? This could potentially influence many of the variables you analyzed (vital signs, lab values). Be sure to explain how selection bias may have influenced your results.
- Some of your variables are statistically similar (i.e. ISS) but clinically different, or vice versa (24 hour bilirubin, chest AIS). Be prepared to explain why these results are or are not meaningful.
- What do the terms safety profile and impact mean in your objectives section. You mention transfusion reaction rate separately, so what other safety and impact factors were you measuring?
- Once again, statistical power is a question. Did you do a power analysis? I worry that a difference of 1 transfusion reaction in 250 patients was used to call whole blood safe.
- How do you know the decrease in post-ED transfusions was due to use of whole blood? Please make sure to summarize the resuscitation given to the two groups while they were in the ED. Did the component group receive fewer units in the ED, thus requiring more afterwards? And vice versa for the whole blood group. Did they get more enroute to the hospital and in the ED?
This was a very interesting abstract. I’m looking forward to hearing many more details when you present.
Reference: Safety profile and impact of low-titer group O blood for emergency use in trauma. EAST 2019 Paper #16.