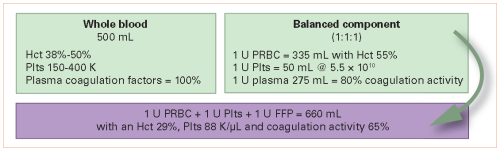
Type AB plasma is considered “universal donor” plasma, as it contains no antibodies to red cells with either A or B antigens on their surface. Unfortunately, only about 4% of the US population have this blood type and can provide the product. Due to this shortage, some trauma centers have decided to use Type A plasma initially for massive transfusion, and switch to type specific plasma once patient blood has been typed and screened.
This works, since only about 13% of the population have red cells with B antigens on the surface. But are there any adverse effects in those patients who receive potentially incompatible plasma? The EAST Multicenter Study group performed a retrospective study using trauma registry and blood bank data from 5 trauma centers. They looked at adult patients who received plasma as part of the massive transfusion protocol (MTP) over a 4+ year period. Incompatible type A plasma transfusion was defined to occur if a patient had either Type B or AB blood.
Here are the factoids:
- There were a total of 1212 patients in the study; 93% were compatible and 7% were incompatible type A initial transfusions
- The usual trauma demographics were seen (young, male) and the average ISS was 25 (they triggered an MTP, remember?)
- By chance, the incompatible group had a slightly higher ISS (29) and penetrating injury rate (45% vs 33%)
- The incompatible group received significantly more plasma during the first 4 hours and during the first day
- There was no difference in mortality sepsis, ARDS, thromboembolic events, or renal failure
- Regression analysis showed that incompatible plasma was not a predictor of mortality or morbidity
- There was one hemolytic reaction and one occurrence of TRALI, both in the compatible group
Bottom line: This is the largest study around on the topic, and it does not show any significant problems (at least the ones that were studied) with giving incompatible plasma in acute trauma. How can this be, you ask? Remember, only the first one or two units (the first MTP pack) is potentially incompatible. Hopefully, by the time the second pack is delivered, the blood has been typed. And these patients are potentially receiving multiple units of typed plasma after the initial transfusion which dilutes the incompatible, and multiple transfusions overall which may blunt their immune response.
This is an important paper that all centers should consider as they update their massive transfusion protocols!
Questions and comments for the authors/presenters:
- The abstract states that 5 centers participated, but the tables only list 4. Please explain this.
- It is not stated explicitly whether all centers used type A plasma initially. Is this the case?
- This is important work! Have any other centers converted to initial use of type A plasma?
Click here to go the the EAST 2017 page to see comments on other abstracts.
Related posts:
- Component therapy vs whole blood: which is better
- Consequences of using incompatible (O+) blood for MTP
- ABC: a quick & dirty way to predict massive transfusion
Reference: Use of incompatible type A plasma transfusion in patients requiring massive transfusion protocol: outcomes of an EAST multicenter study. Paper #16, EAST 2017.