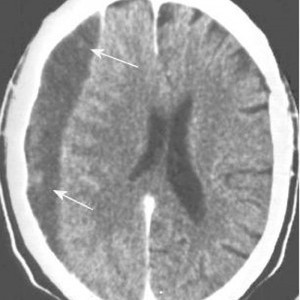
Patients with traumatic brain injury (TBI) severe enough to cause bleeding are usually admitted to the hospital for observation and in many cases, repeat CT scanning. Those with small intracranial hemorrhages (ICH) may experience progression of the bleeding, and a small percentage of cases may need operative intervention (1-3%). Questions we typically face are, how long should we watch for progression, and how often should we scan?
A retrospective cohort study was carried out at UMD-NJ, looking for answers for a specific subset of these patients. Specifically, they had to have a mild blunt TBI (loss of consciousness and/or retrograde amnesia, GCS in the ED of 13-15) and a positive head CT. They classified any type of hemorrhage into or around the brain as positive.
During a 3 year period, 474 adults were enrolled but only 341 were eligible for the study. They were excluded due to previous injury, presence of a mass (not trauma), need for immediate neurosurgical intervention, or failure to get a second CT scan. The authors found:
- 7% of patients were taking anticoagulants! This is surprisingly high. Interestingly, 15 were subtherapeutic, 3 were therapeutic and 2 were supratherapeutic.
- Subarachnoid hemorrhage was the most common finding on CT (54%). Intraparenchymal hemorrhage was next most common (48%) Many patients had more than one type of bleed.
- The injury worsened between the first and second scans in 31% of patients. This number increased to 46% in patients taking anticoagulants.
- About 97% of bleeds stopped progressing by 24 hrs post-injury.
Bottom line: Most centers are probably overdoing the observation and repeat scan thing. More than two thirds of bleeds are stable by the first scan (first and second scans identical), and nearly all stop progressing within 24 hours. It’s very likely that patients who are not on anticoagulants and who have a stable neuro exam and stable symptoms can get just one scan and 24 hours of observation. Persistent headache, nausea, failure to ambulate well, or other symptoms warrant a repeat scan and longer observation.
Related posts:
Reference: The temporal course of intracranial haemorrhage progression: How long is observation necessary? Injury 43(12):2122-2125, 2012.