This abstract falls into the “interesting, but how can we use this bit of information” category. We’ve known that transfusing packed red cells raises nosocomial infection rates for at least 15 years. The group led by MetroHealth in Cleveland combined forces with the Vanderbilt trauma group to re-look at their data from the PAMPer trial with respect to trauma patients.
The PAMPer trial (Prehospital Air Medical Plasma) examined the effect of tranfusion of two units of plasma in the air ambulance on mortality, transfusion need, and complications. Half of the patients got plasma plus standard care, and the other half standard care alone.
This abstract uses PAMPer trial data to examine the impact of giving any amount of blood on nosocomial infection in these patients. These infections included pneumonia, bloodstream infection, C Diff colitis, empyema, and complex intra-abdominal infection.
The group retrospectively analyzed the prospectively collected PAMPer data and used logistic regression models to test for an association.
Here are the factoids:
- A total of 399 patients with the usual trauma demographics were included (younger male, moderately injured, blunt mechanism)
- Ten percent of patients died, and 23% developed nosocomial infections
- Pneumonia was by far the most common complication (n=67) with all others in the low teens or below
- Although only two thirds of patients received plasma, 80% were given PRBCs and 27% received platelets
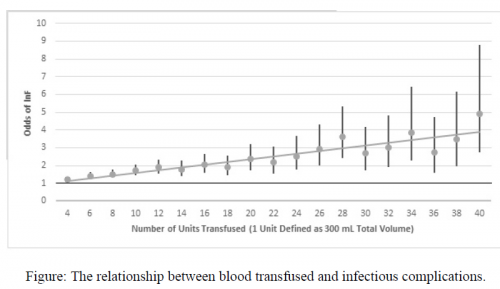
- Patients who received any amount of packed cells had a 2.3x increase in nosocomial infections, and the number given increased the rate of nosocomial infection (1.06x)
The authors concluded that patients in the PAMPer trial who received at least one unit of blood “incurred a two-fold increased risk of nosocomial infection” and that this risk was dose dependent.
My analysis: The biggest obstacle for me to buy into this work is the enrolled patient group. Studies in which you borrow someone else’s data are always a bit problematic. You have no control over the variables, as they’ve been determined by someone else.
In this case, the dataset could only be controlled for age, sex, and ISS. But what about all the other stuff that might have an impact on infections? Things like pulmonary injury, the 20% of patients who had penetrating injury, and severe TBI patients with their propensity to develop VAP.
The odds ratios of the associations were a bit on the low side. Sure, the overall nosocomial infection odds ratio was 2.37 but the confidence interval was 1.14 to 4.94. This is very wide and it means that the odds could have been anywhere from 1.14x to almost 5x. This suggests that the study group may not have been large enough to give us a clear picture. And the odds ratio for number of PRBC units vs infection was only 1.06 with a tighter confidence interval. So even if it is present, this association is very, very weak. I like to see ridiculously large odds ratios when reviewing observational studies like this where the input data is constrained.
My final comment on this study deals with its utility. These are trauma patients. They are bleeding. We’ve known that transfusions may increase the nosocomial infection rate in critically ill patients for at least 15 years. But we will still have to give the patients blood. So what are we to do?
Here are some questions for the authors and presenter:
- Please comment on the limitations you faced using the PAMPer dataset. Were you satisfied with the range of variables available? Which additional ones would you have liked to work with?
- Do you feel that the 399 patients provided enough statistical power for analysis? The confidence intervals are large and very close to the OR=1 line.
- What should we do with your conclusions? Can we translate this into clinical practice?
One final note: the patients did not “incur increased risk.” Rather, there was an association with increased risk of infection. We really don’t know if it was from the blood or something else that was not recorded in the PAMPer dataset.
Reference: Dose-dependent association between blood transfusion and nosocomial infections in trauma patients: a secondary analysis of patients from the PAMPer trial. EAST 2021, Paper 3.