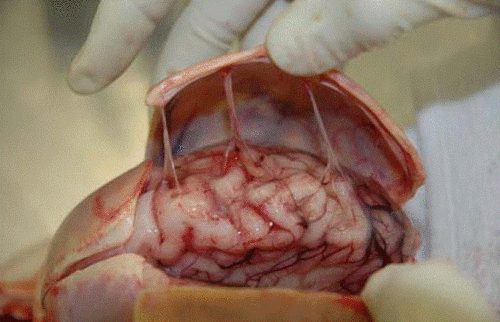
The books all say “transport the patient with an impaling object in place” and “only take the impaling object out in the operating room.” Is this realistic? How do you actually take that knife out?
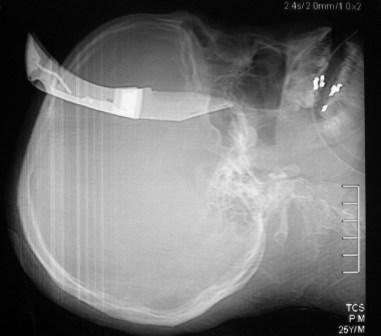
First, you need to decide if the patient belongs in the OR right now. Are they hemodynamically unstable? Is there obvious arterial bleeding? If so, don’t dawdle. Proceed to the operating room and surgically expose the problem completely.
If the patient is safe to stay in the ED, do what you need to figure out the exact anatomy of the wound (and object). This may involve imaging, usually CT scan. Once the exact position of the object is understood, build an anatomical picture of the situation in your mind. What named arteries might be involved? What other vital structures?
Given this anatomic information, a decision can then be made regarding the best location for removal. The majority of the time, this will be in the operating room. It is best to obtain optimum surgical exposure prior to pulling it out. In the abdomen, this is easy. However, some areas (skull, sinuses) are tricky and may not require exposure of the end of the tract. Visualization of the remaining hole(s) is key so that bothersome bleeding can be recognized immediately.
The object should be grasped firmly and carefully and removed in one smooth motion. Visual monitoring for five minutes will virtually eliminate the presence of bleeding. If it does occur, then deeper exploration is warranted. In the awake patient, I generally push gently on either side of the entry point prior to and during the pull to provide some sensory distraction. Then I hold pressure on the site for 5 minutes (no peeking) to assure myself that there is no bleeding.
And don’t forget the forensics! Let the police photograph the patient. Handle the object carefully so as not to disturb any fingerprints. Place it carefully in a paper bag, labelled appropriately. And always make sure that a chain of evidence form is properly filled out so it and the object itself can be handed over to the proper authorities.