Clinical clearance of the cervical spine is a standard of care. It is usually the first method to determine if there might be an injury in patients who are awake, cooperative, and don’t have other painful distracting injuries. But appreciation of pain may be different in elderly patients, and they will frequently not notice pain from some injuries. Could this possibly impact clearance of the cervical spine?
A group at Iowa Methodist performed a retrospective review of patients > 55 with diagnosed cervical spine fractures over a four year period. They were considered to have an asymptomatic injury if they did not complain of pain, or of tenderness to palpation.
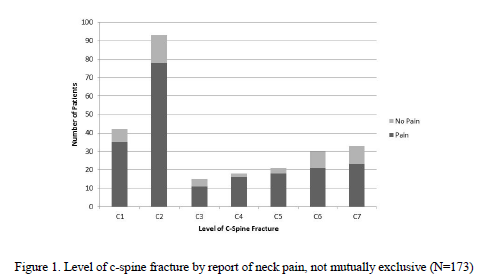
Here are the factoids:
- A total of 173 elderly patients presented with a cervical spine injury during the study period
- 38 of them (22%) were asymptomatic
- The asymptomatic patients tended to have higher injury severity (ISS 15 vs 10), have a significant injury in another body region (71% vs 47%), and stayed in the hospital longer (7 days vs 5)
- A third of patients had multiple cervical fractures (symptomatic or asymptomatic?)
- C2 was the most common fracture level
Bottom line: I have witnessed this phenomenon myself. Not all of our elders perceive pain the same way younger patients do. This study shows that it is a very significant problem. Most of the previous papers and the only review I could find do not separate out the elderly when making cervical clearance recommendations. We will probably have to develop some specific criteria to determine when a CT scan is necessary in the asymptomatic elderly patient. In the algorithm used at my hospital, age > 65 is already used to bypass clinical clearance. Looks like I’ll have to drop that to 55!
Questions and comments for the authors/presenters:
- Since they were asymptomatic, how do you know that you didn’t miss any patients?
- Do you have a practice guideline for cervical spine evaluation? Has it changed based on your study?
- Be sure to break your data down by mechanism of injury for the presentation. Were there more asymptomatic patients from falls rather than car crashes? Associated fracture patterns for each mechanism?
- What do you now recommend for clearance?
- Suggestion: change your title to “cervical spine fractures”, not “neck fracture”.
Click here to go the the EAST 2017 page to see comments on other abstracts.
Related posts:
- VIDEO: How to clinically clear the cervical spine
- Practice guideline: cervical spine clearance in the alert patient
Reference: Asymptomatic neck fractures: current guidelines can fail older patients. Paper #8, EAST 2017.

