Pelvic Fractures: OR vs Angio In The Unstable Patient
One of the cardinal rules of trauma care is that hemodynamically unstable patients can only go the the operating room from the ED. No trips to CT, xray, etc. Trauma professionals occasionally try to make exceptions to the rule, but it usually doesn’t work out.
Well, what about the patient with severe pelvic fractures who is or becomes unstable? Pelvic fracture bleeding is not always easy or even possible to control in the OR, and angiography offers a way to identify and stop the bleeding, right?
The trauma group at Ryder in Miami did a lengthy (13 year) retrospective review of their experience with these patients. They looked at every patient who underwent angiography, then identified the subset that went to the OR followed by angiography. There were 134 angio patients and 49 OR to angio patients on whom they based their analysis. Obviously, there is plenty of opportunity for bias in this study, and many of the study patients identified had to be excluded due to incomplete records.
Patients who went to the OR first tended to have similar injury severity but were sicker than the angio alone group. Crystalloid and blood resuscitation volumes were significantly higher in the OR group as well. Most of these patients underwent a laparotomy, and 64% had active intra-abdominal bleeding. None died in OR, and most were left with a damage control abdominal closure.
In the angio group, there were really 2 subsets: angio alone, and angio followed by OR. Mortality in the angio alone group was similar to the OR-angio group. But deaths skyrocketed in those who went from angio to OR (67% vs 20%). This is likely due to them failing angiographic management of bleeding. Three patients died in the angio suite.
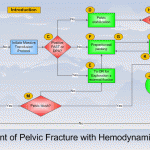
Bottom line: There’s a lot of data in this paper, and some of the results can be explained by selection bias. However, they appear to support algorithms released by EAST and the WTA (see diagram above). In general, a trauma patient with severe pelvic fractures and hemodynamic instability needs to go to OR to identify and treat any source of intra-abdominal bleeding. If pelvic bleeding remains a problem, preperitoneal packing may be considered, followed by a trip to angio at that point. The rule that unstable patients should only go to OR (or an ambulance bound for a trauma center if there is no OR) still holds!
Reference: Operating room or angiography suite for hemodynamically unstable pelvic fractures? J Trauma 72(2):364-372, 2012.
Quiz: There is just one extremely rare reason that I know of to move to CT with a hemodynamically unstable trauma patient. Leave a comment with your guess.