REBOA (resuscitative endovascular balloon occlusion of the aorta) is one of the relatively new toys in our trauma toy chest. Although it’s been used for decades by vascular surgeons, believe it or not it only made the jump into the trauma world less than 10 years ago.
The original catheters used during the early days, and primarily in swine models, required a 15 French sheath for insertion. As might be expected, insertion of these huge sheaths into the common femoral artery can cause significant vascular injury. Equipment manufacturers have been steadily reducing the size of the REBOA catheter, first to 12 Fr and then to the 7 Fr size commonly used today.
The surgery group at the London Health Sciences Center in London, Ontario, Canada performed a pilot study of a new and much smaller sheath and REBOA catheter. It is made by Front Line Medical Technologies, also located in London. This was a proof of concept for the device and was performed in seven neurological death organ donors prior to their donation.
The kit consists of a 4 Fr sheath introducer with a 21 gauge needle and a guidewire, plus the REBOA catheter itself. Here is an image of the catheter:
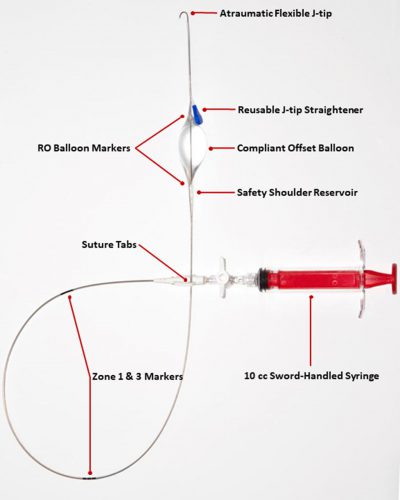
This catheter includes several innovations not found in current catheters used in the US. I will do a side by side review of these later this week.
Here are the factoids:
- Seven organ donors were studied after appropriate consent from the hospital IRB, organ procurement agency, organ donor procurement team, and family
- A single general/vascular surgeon performed all insertions
- A left sided arterial line using the 4 Fr sheath was inserted for monitoring before the procurement began
- A right sided 4 Fr sheath was inserted for catheter insertion after the procurement incision was made
- Average sheath insertion time was 48 seconds, and deployment time for the catheter was an average of 70 seconds (max time was 105 seconds)
- Occlusion was confirmed by the left femoral arterial pressure monitor and by palpating the aorta below the baloon
Bottom line: This was a very simple study of the feasibility of using a smaller REBOA catheter. It measured both ease of insertion and presence of full occlusion. This is an exciting study, because there is the potential for easier insertion and fewer vascular complications at the insertion site. Obviously, these factors are not yet known, and only further work will make this clear.
Nonetheless, easier and safer insertion has the potential to increase the use of REBOA. This will allow us to get quicker answers to the nagging questions about whether it is actually a valuable resuscitation tool and help us figure out how and in whom it is best used.
Reference: Size matters: first-in-human study of a novel 4 French REBOA device. Trauma Surgery & Acute Care Open 2021;6:e000617. doi: 10.1136/tsaco-2020-000617

