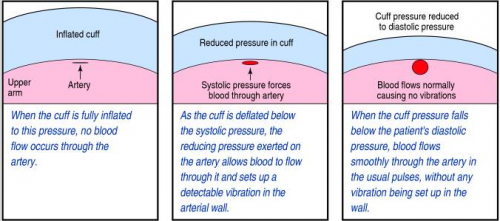
Yesterday, I described how the typical automated oscillometric blood pressure cuff works. We rely on this workhorse piece of equipment for nearly all pressure determinations outside of the intensive care unit. So the obvious question is, “is it accurate?”
Interestingly, there are not very many good papers that have ever looked at this! However, this simple question was addressed by a group at Harvard back in 2013. This study utilized an extensive ICU database from 7 ICUs at the Beth Israel Deaconess Medical Center. Seven years of data were analyzed, including minute by minute blood pressure readings in patients with both automated cuffs and indwelling arterial lines. Arterial line pressures were considered to be the “gold standard.”
Here are the factoids:
- Over 27,000 pairs of simultaneously recorded cuff and arterial line measurements from 852 patients were analyzed
- The cuff underestimated art line SBP for pressures at or above 95 torr
- The cuff overestimated SBO for pressures below 95 torr (!)
- Patients in profound shock (SBP < 60) had a cuff reading 10 torr higher
- Mean arterial pressure was reasonably accurate in hypotensive patients
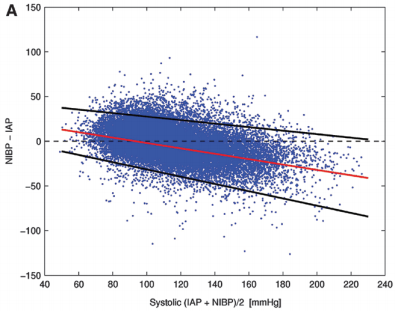
Bottom line: The good, old-fashioned automated blood pressure cuff is fine for patients with normal pressures or better. In fact, it tends to understimate the SBP the higher it is, which is fine. However, it overestimates the SBP in hypotensive patients. This can be dangerous!
You may look at that SBP of 90 and say to yourself, “that’s not too bad.” But really it might be 80. Would that change your mind? Don’t get suckered into thinking that this mainstay of medical care is perfect! And consider peeking at the mean arterial pressure from time to time. That may give you a more accurate picture of where the patient really is from a pressure standpoint.
Related posts:
- What do I do: small hospital, unstable patient
- Can I take a hypotensive patient to CT?
- Hypotensive patients and CT scan – Part 2
Reference: Methods of blood pressure measurement in the ICU. Crit Care Med Journal, 41(1): 34-40, 2013.