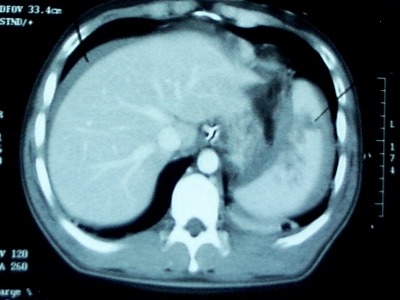
Angioembolization of the spleen (AES) is part of our armementarium in the management of spleen and liver trauma. However, there are no good guidelines to help us decide exactly which patients would benefit from it. An abstract to be presented at the EAST meeting in January 2013 gives us a little more information on the actual benefits of this procedure.
The authors did a retrospective review of the management of blunt splenic injury at four busy Level I trauma centers. They looked at 1275 injured patients over a 3 year period. Here are the interesting tidbits from the study:
- There was considerable variation in the use of AES at the 4 centers, ranging from 1% of patients to 14%. This should be no surprise because there is no real guidance available yet.
- There was also a large degree of variation between the number of initial splenectomy performed at these centers
- Centers that used AES more frequently had lower initial splenectomy rates
- Patients at centers with high AES rates were 3 times more likely to leave with their spleen intact
Bottom line: This abstract correlates with my own personal experience: judicious use of angioembolization saves spleens. The real question is about which patients are best served by it. Our protocol is to strongly consider it in all high grade spleen injuries (Grade 4 and 5), and to always do it if a blush or extravasation is present. Our success rate for nonoperative management currently stands at about 94%.
Related posts:
Reference: Variation in splenic artery embolization a spleen salvage: a multicenter analysis. EAST Annual Scientific Assembly, Paper #1, to be presented January 2013.