The protests in cities across the country continue. Many are peaceful, but not all. In some cases, police have resorted to “non-lethal” weapons to control and disperse crowds.
Although these weapons are called non-lethal, that’s not entirely true. The projectiles, gases, and powders that are being used all have some degree of morbidity and mortality. They are certainly less so than traditional projectiles (bullets), but serious and fatal injures can and do occur.
One item that is talked about in the news is the rubber bullet. What are these, exactly? The generic term is a “kinetic impact projectile” (KIP). It encompasses a variety of objects that are not designed to penetrate flesh like a regular bullet. They can be bullets, beanbags, sponges, pellets, and other odds and ends.

And the so-called “rubber bullet” isn’t even necessarily made of rubber. It can be plastic, metal, rubber, or other substances.
There is very little published data on injuries caused by KIPs. Because of their odd shapes, they tend to tumble when they are fired. This decreases aiming accuracy substantially when the target is distant. They are designed to be aimed at the lower extremities. However, if the aim is too high or the round is fired at close range, it can be lethal.
Here are some typical injuries that have been descirbed:
- Subdural and intraparenchymal hematomas
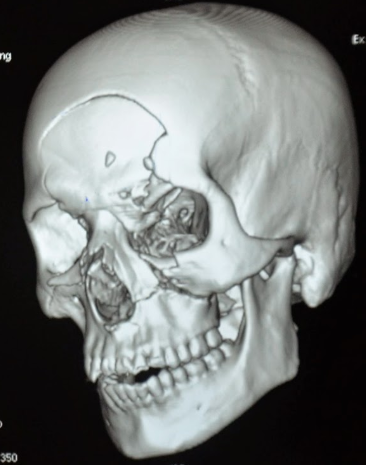
- Skull and facial fractures
- Eye injuries leading to blindness (this happened to a photographer in Minneapolis last week)
- Rib fractures and pulmonary contusions
- Spleen laceration
- Blunt intestinal injury
Here’s a video from one of the manufacturers that shows the amount of target deformation caused by a sponge tipped bullet. Very impressive.
(Tumblr viewers please click here to view video)
Bottom line: Although they sound relatively innocuous, kinetic impact projectiles of any kind are far from it. If you are called to treat a patient who has been shot with one, be sure to do a very thorough evaluation. Most head, neck, and torso injuries should undergo CT scanning to delineate deep or occult injuries in detail. In-hospital observation of torso injuries is warranted, as well as a good tertiary exam.