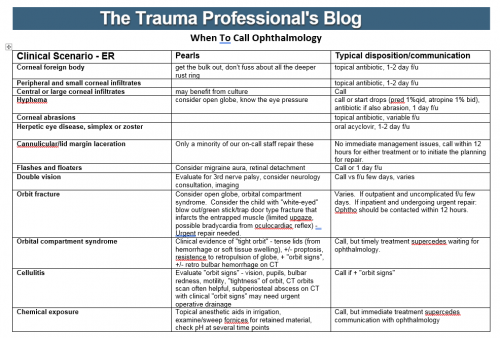
Early tracheostomy is generally accepted to be a good thing in critically injured patients. A number of papers have shown that it decreases ventilator days and hence ICU and hospital days, and also reduces pneumonia rates, sedation requirements, and saves quite a bit of money.
However, there has been one problematic group in whom critical care surgeons are often forbidden to do an early trach: patients with a recent anterior cervical fusion. These patients typically have unstable cervical fractures, with or without a concomitant spinal cord injury. The spine surgeons argue that doing a trach “too soon” leads to a higher infectious complication rate due to the proximity of the trach to their anterior surgical wound.
But is this really true? The trauma/critical care program at Thomas Jefferson University in Philadelphia looked at their experience. They are a federally sponsored spinal cord injury center, and have a vast experience compared to most trauma centers. They reviewed their experience over a 16 year period. Typically, they performed all of their tracheostomies within 10 days, so they arbitrarily defined “early” as within 4 days, and “late” as > 5 days after the cervical procedure.
Here are the factoids:
- A total of 98 patients with tracheostomy after anterior fusion were included in the study, some of whom also underwent a concomitant posterior fusion
- 39 cases were “early”, within 4 days of the anterior fusion procedure, and 59 were “late”
- Average time to fusion in the early group was 2 days, and 10 days in the late group
- There were no wound infections in the early group
- There were 5 wound infections in the late group, and 4 of them involved the posterior fusion site(!)
- The only infection of the anterior fusion site occurred in a late patient who suffered an esophageal perforation from the fusion hardware
Bottom line: Although the numbers are still small after 15 years of data, it’s probably the best we will ever get! It is clear that an anterior fusion wound is safe in these procedures. I am at a loss as to why the posterior fusion wounds tend to get infected, though. But the next time your spine surgeons balk about doing an early trach in one of their anterior fusion, show them this paper!