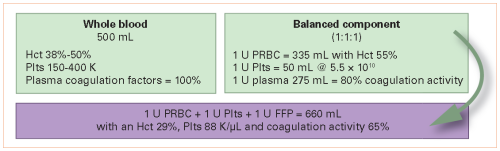
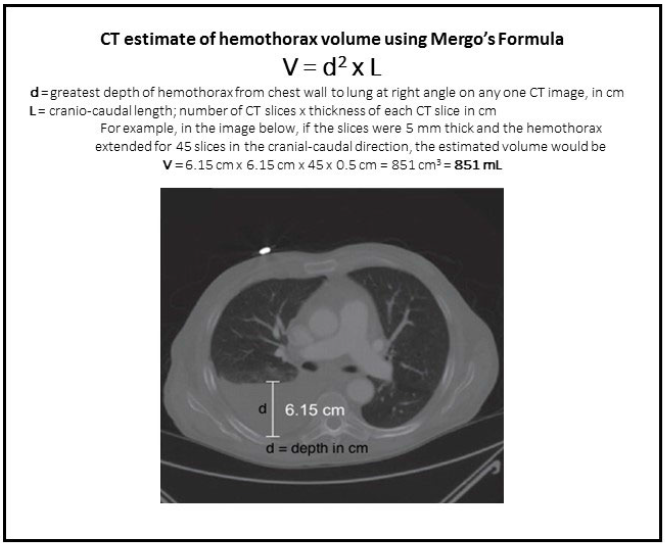
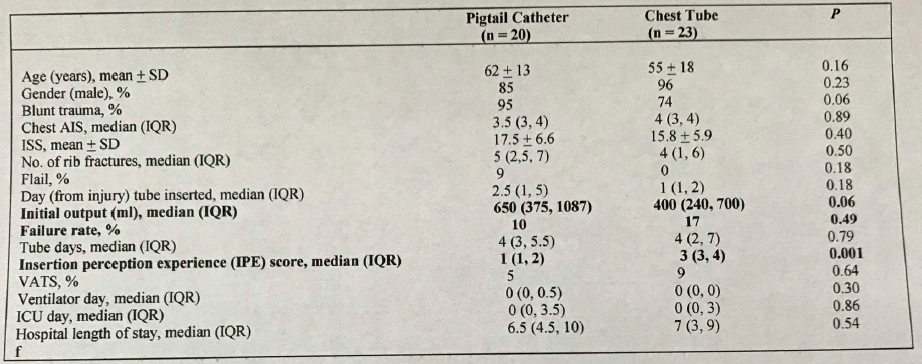
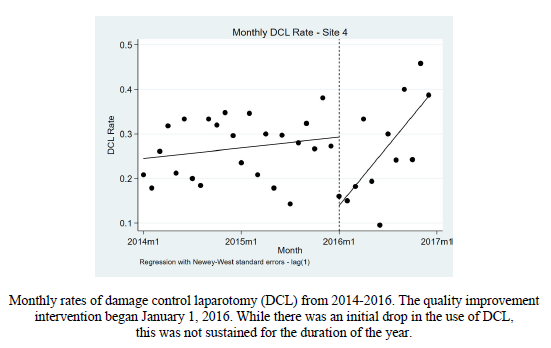
Trauma centers verified by the American College of Surgeons (ACS) (and most states who perform their own designation visits) are required to engage in trauma prevention activities. Furthermore, ACS centers are required to provide prevention programs based on identified local needs. Frequently, trauma professionals see a pattern of injury in the patients they treat. This generally stimulates a search through their trauma registry. Reviewing registry data is the most direct way of identifying and confirming injury patters specific to the local population.
The next abstract for review describes the process and outcomes of such a project from a Level II center in Fort Walton Beach, Florida. They noted a pattern of diving injury and high cervical fractures. This was confirmed using 2016 registry data. Admitted patients were intensive resource users, with 71% requiring ICU admission and operative fixation, and nearly half requiring rehab admission upon discharge.
Based on this, they developed a “Think Before You Dive” program with posters, signs, swag (a custom koozie), a trifold brochure, and magnets with diving and water safety tips. Posters and flyers were provided to local business, and magnets were placed in hotel rooms in the area. One time-share company even placed a hard stop in their registration process so that visitors had to acknowledge the safety message.
What’s a koozie, you ask? I didn’t know the technical term for this, but here’s a picture:

Here are the factoids:
- There was a reduction of 100% in cervical spine injuries, and 24% of all water-related incidents in the targeted area
- All remaining diving/high-cord injuries came from outside the target area
- It was estimated that costs were reduced by $1.2 million
As you can see, this is not the typical hard research paper usually provided at most scientific meetings. However, it is very important that this kind of information is presented, as it has the potential for impact on the other clinical research.
Here are some questions for the authors and presenter to consider in advance to help them prepare for audience questions:
- How did you recognize the problem initially? Was it a pattern picked up by humans? Which ones (nurses, trauma physicians, therapists/rehab, others)?
- Why did you think that your prevention approach would be effective?
- Provide some details on how you convinced businesses to carry your message. Was there any resistance, and what were their arguments? How did you overcome it?
- Show us the numbers. Although it may be difficult to show statistical differences in patient numbers, cost savings is important as well. Show the patient numbers pre- and post-intervention for the cluster area and outside of it.
- Define how you arrived at your cost savings numbers. How do the previously published economic numbers relate to costs at your own center and those reported in this study?
I believe that this is important information, and will help many other centers properly design their own trauma prevention programs!
Reference: Using the trauma registry to guide your injury prevention programs. EAST 2019 Paper #18.