Well, it’s that time of year again! The annual American Association for the Surgery (AAST) is just a few weeks away. Starting today, I will begin reviewing some of the interesting abstracts (to me, at least) that will be presented. I’ll give my analysis and perspective, and usually provide some questions for the presenters that they may face during the live meeting. Enjoy!
I’ll start with abstract #1. This one is from the AAST Prospective Observational Vascular Injury Trial (PROOVIT). The group was established to create an aggregate database of information on the presentation, diagnosis, management (acute and definitive), surveillance and outcomes following vascular trauma. It manages a registry that collects a wide variety of data on assorted vascular injuries.
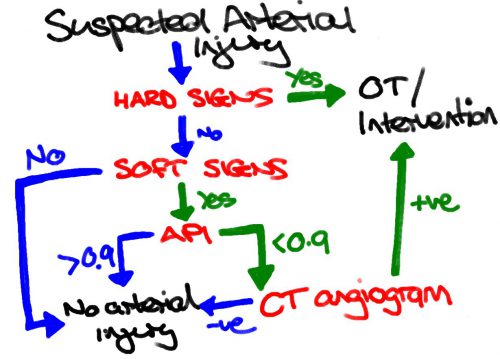
This study re-examines our use of “hard signs” to diagnose vascular injury. Back in the day, we had “hard signs” and “soft signs.” Hard signs were fairly obvious indicators of serious injury, such as pulselessness, ischemia, pulsatile bleeding, expanding hematoma, or a thrill or bruit. Soft signs were a bit less harsh: history of arterial bleeding, diminished pulse, stable hematoma, or an injury in proximity to the vessel.
In the old days, any hard sign of vascular injury was a hard indication to proceed directly to the OR for exploration and repair. However, the authors argue that in this day and age of advanced imaging and noninvasive treatment, maybe hard signs aren’t as hard as they used to be. They postulated that distinguishing between hemorrhage and ischemia would be more important in determining management of these injuries.
They focused on femoral and popliteal artery injuries, searching the database for classic hard vs soft signs, and newer ischemic (absent or diminished pulses, frank limb ischemia) vs hemorrhagic signs (overt hemorrhage, expanding hematoma, hypotension). They examined the presentation, pathology, treatment and outcome in 521 patients in the registry
Here are the factoids:
- Hard signs occurred in 386, and 35% underwent CT angio instead of moving directly to OR
- Soft signs occurred in the remaining 175, and 39% went to the OR without any further imaging
- When using hemorrhage (HEM) vs ischemia (ISC), there were significant differences in mechanism (more penetrating in HEM), incidence of concomitant vein and nerve injury (higher in HEM), transection (higher in HEM), occlusion (higher in ISC)
- For diagnosis and management, HEM was more likely to get intervention sooner, without imaging, using ligation or primary repair
- ISC was more likely to undergo endovascular repair
- HEM patients used a little more blood and had a higher mortality rate
- Amputation rates, lengths of stay, and graft outcomes were the same
The authors concluded that the old hard vs soft signs paradigm no longer works, and suggest that using hemorrhage vs ischemia in now more useful.
Bottom line: This is a simple, straightforward descriptive study of five years of vascular injury of the proximal lower extremity. It certainly paints the picture that the old paradigm doesn’t work as well as it used to. About a third of patients with hard signs had preop imaging, and about the same number with soft signs went straight to OR.
The major drawback is that this is what I call a “how we do it study.” The results are largely dependent on the predominant practices at the participating centers. What if most of the centers that chose to participate are much more likely to use diagnostic imaging first, or go straight to OR first? And that centers that obeyed the classic hard vs soft signs paradigm steered clear? That could skew the results in this study.
This is a very thought-provoking paper. I’m looking forward to hearing more of the details at the meeting. I’ll be in the front row!
Questions for the authors and presenter:
- Why did you focus only on femoral and popliteal injuries? What should be do about the others?
- What were the “demographics” of the participating centers? What trauma center level, academic or not, urban or rural? All of these could have a significant impact on your numbers.
- What was the duration of experience captured in the database? Are you able to see changes in preop eval or straight to OR practice over the years?