More On Treating Pneumothorax With Oxygen
One of my readers has pointed out that, yes, the evidence for using O2 to treat pneumothorax is poor, but practice and standard of care are not always driven by evidence. He also pointed out that it’s not really fair to condemn the use of this modality if there isn’t specific evidence showing that it’s bad. In other words, doing something that seems benign is okay if we can’t show that it’s harmful or at least prove that it’s actually benign. I don’t agree.
My point is that no intervention is truly benign. There are always potential complications for the things we do as physicians, sometimes physical, sometimes psychological. Putting a patient on O2 seems safe. But if used as a treatment for pneumothorax, it means hospitalization (which costs a lot of money), an IV (which could get infected), exposure to a lot of sick people (read MRSA and other fun bugs), lying in bed a lot more than at home (DVT), and on and on.
If the pneumothorax does not interfere with function and the patient has decent pulmonary health, why not send them home with reassurance and get a followup chest xray at some point to confirm resolution? If it does cause physiologic problems, or they have pulmonary disease and are likely to develop complications such as pneumonia, then admit for the least invasive treatment to quickly get it out (pigtail type catheter).
Since this topic just won’t seem to die, I’m going to try to kill the last papers I’m aware of on this topic today and tomorrow. Today’s was published in a pediatric surgical journal (!), and it’s another rabbit study. This one adds a wrinkle to the one I discussed yesterday. Not only did they inject air to create a pneumothorax (20cc this time), they punctured the pleura with a needle to create an air leak to simulate a real clinical problem.
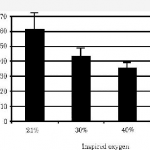
They saw the same trend as posted yesterday, although the times were longer. Once again, resolution was measured with chest xray (performed every 12 hours this time). Unfortunately, 7 of the 27 rabbits used in each group died, leaving only 6 or 7 in each of 3 groups for analysis (room air, 40%, 60% O2). Even with wide standard deviations, the authors claimed significant differences in recovery.
Same problems as yesterday, particularly with how resolution of pneumothorax is determined. And don’t use rabbits! A bigger issue is that this is not really a clinically relevant model. First, creating an air leak would defeat the overall purpose of giving high O2 concentrations. If 60% O2 leaked into the pleural space, there would be less nitrogen to wash out so one would think that resolution would take longer. And no one would consider treating a patient with an air leak without some type of drainage device for fear of a tension pneumothorax.
Bottom line: Still not enough evidence to support this seemingly benign treatment. Tomorrow I’ll look at the (hopefully) last paper on the topic since the beginning of time, published in 1971.
Related posts:
Reference: Supplemental oxygen improves resolution of injury-induced pneumothorax. J Pediatric Surg 35(6):998-1001, 2000.
Thanks to Jonathan St. George for his comments on yesterday’s post!