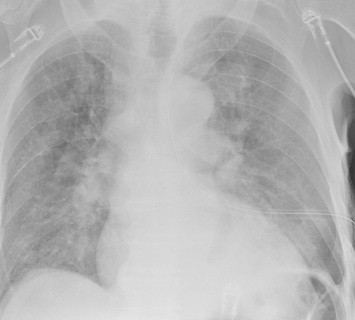
There is an art to deciding when to place a chest tube for either hemothorax or pneumothorax. For the most part, the trauma professional examines the imaging and then uses some unknown internal metric to declare that it is “too big.” Then it’s time to insert some type of chest drain.
There have been attempts over the years to make this decision more quantitative. One of the better-known ones is the 2-cm rule for pneumothorax. If the distance from the chest wall to the lung on the chest x-ray is >2cm, it is “too big.”
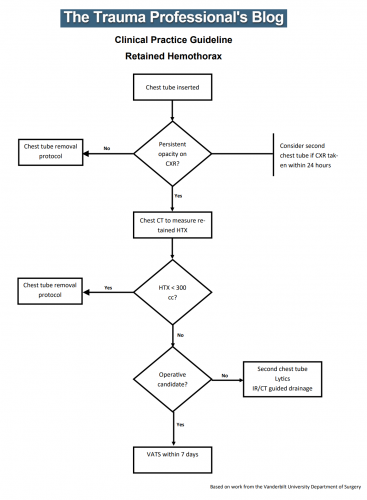
But what about hemothorax? The Medical College of Wisconsin trauma group performed a retrospective review of 391 patient charts to test a new 300cc rule defining when a hemothorax is “too big.” This guideline was implemented in 2018-2019, and patients presenting before implementation were compared to those arriving after.
The 300cc threshold is determined by using Mergo’s formula for calculating the volume of a square prism. Obviously, this requires a CT scan for calculation, so patients who had a tube placed before scanning or did not have one were excluded. They were also excluded from the study if their pneumothorax met the 2-cm rule. The authors studied how many patients could be observed, how many needed tube drainage, observation failure, and later need for a VATS procedure or thoracotomy.
Here are the factoids:
- About 60% of the study group was admitted after the new criteria were implemented, and both groups were demographically similar
- After implementation, the number of patients that were just observed increased significantly from 52% to 71%
- Of course, this means that the number of chest tubes inserted was significantly less (42% vs. 61%)
- There was no difference in observation failure (delayed placement of a tube), 18% vs. 24%
- There were also no differences in pulmonary complications, 30-day readmissions, or 30-day mortality
- The average ICU and hospital length of stays were significantly shorter as well
The authors concluded that implementing their 300cc guidelines correlated with decreased length of stay and no increase in failure or complication rates.
Bottom line: Although this is a relatively small series, the differences between the groups quickly achieved significance. There are three major questions that I have. First, how was the 300cc threshold arrived at? Was this borne of clinical judgment, or did some previous work suggest it?
My next question has to deal with the accuracy of the volume calculation. Mergo’s formula was used to determine the volume of a rectangular solid. As we all know, hemothoraces and pneumothoraces are not cubes. They can be very irregular and influenced by patient position. However, I did find a paper from the University of Florida that found the correlation coefficient between the volume calculated by Mergo’s formula vs. using 3-D software estimation was 0.9, which is excellent. So this approximation appears to be a very good one.
Finally, using the 300cc rule is predicated on getting a CT scan. Does every patient need a chest CT? Part of the resuscitation process for major trauma involves obtaining a chest X-ray. The obviously large hemothorax can justify inserting a chest tube at that point. But the reality is that most of these patients do go on to chest CT, so this is a minor change in practice for most.
Although I love to see confirmatory studies before practice changes, this one study can lead us to change our practice guidelines now. It is a relatively minor one and will allow us to avoid placement of a few more chest tubes and to shave off a few days of hospital stay. The logical follow-up study for the authors is to extend the post-discharge window for complications to 60 or 90 days to ensure that delayed procedures were not required in the observation group.
References:
- Implementing the 300-cc rule safely decreases chest tube placement in traumatic hemothorax. AAST 2023 Plenary paper #22.
- New formula for quantification of pleural effusions from computed tomography. J Thorac Imaging. 1999 Apr;14(2):122-5.