There’s a lot of confusion about subdural pathology after head trauma. All subdural collections are located under the dura, on the surface of the brain. In some way they involve or can involve the bridging veins, which are somewhat fragile and get more so with age.
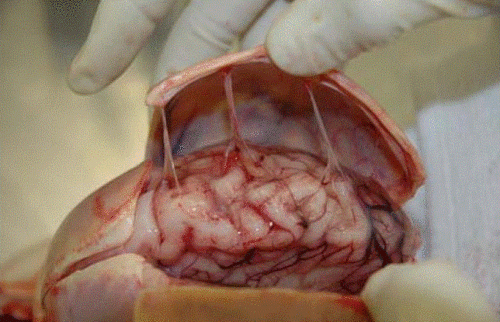
Head trauma causes a subdural hematoma by tearing some of these bridging veins. Notice how thick the dura is and how delicate the bridging veins are in the image below.
When these veins tear, bleeding ensues which layers out over the surface of the brain in that area. If the bleeding does not stop, pressure builds and begins compressing and shifting the brain. A subdural hematoma is considered acute from time of injury until about 3 days later. During this time, it appears more dense than brain tissue.
After about 3-7 days, the clot begins to liquefy and becomes less dense on CT. Many hematomas are reabsorbed, but occasionally there is repeated bleeding from the bridging veins, or the hematoma draws fluid into itself due to the concentration gradient. It can enlarge and begin to cause new symptoms. During this period it is considered subacute.
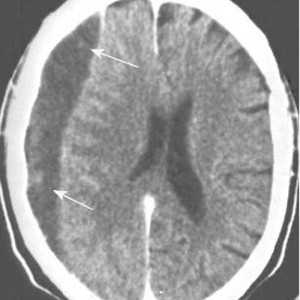
It moves on to a more chronic stage over the ensuing weeks. The blood cells in it break down completely, and the fluid that is left is generally less dense than the brain underneath it. The image below shows a chronic subdural (arrows).
Hygromas are different, in that they are a collection of CSF and not blood. They are caused by a tear in the meninges and allow CSF to accumulate in the subdural space. This can be caused by head trauma as well, and is generally very slow to form. They can lead to slow neurologic deterioration, and are often found on head CT in patients with a history of falls, sometimes in the distant past. CT appearance is similar to a chronic subdural, but the density is the same as CSF, so it should have the same appearance as the fluid in the ventricle on CT.
Related posts: