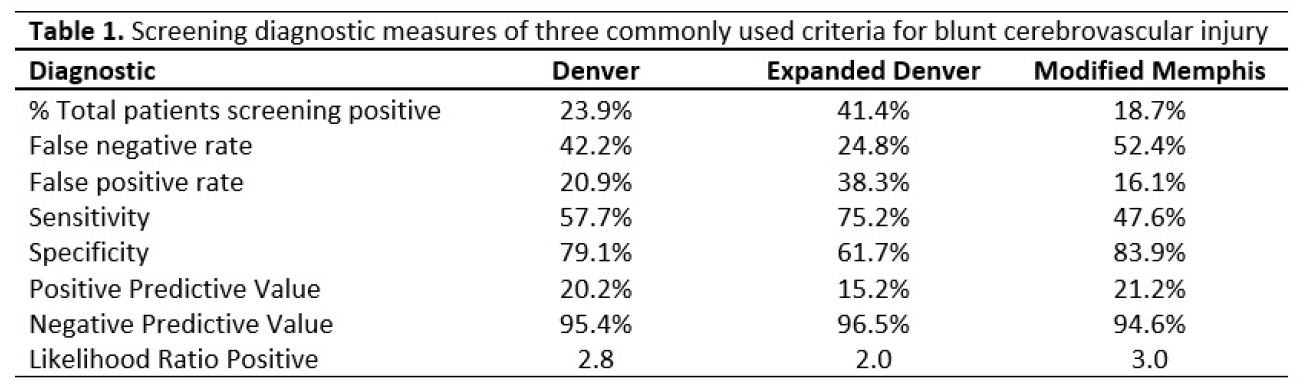
The next abstract is an interesting demonstration of the use of technology is trauma resuscitation. Pretty much all technology imaginable. It details the use of a “hybrid ER” room, which combines resuscitation space with all sorts of imaging and even interventional angiographic procedures. Here’s an image of the room when it was first written about in 2012.
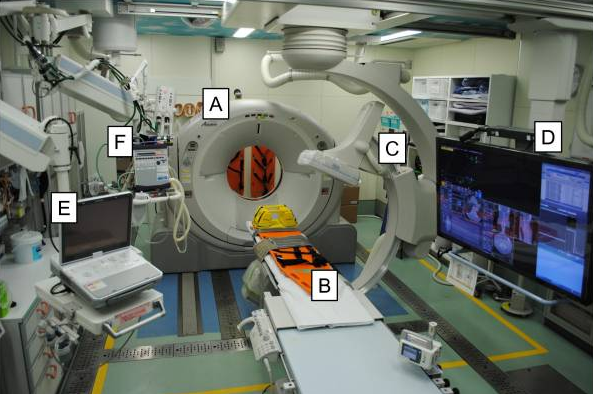
A = CT scanner B = CT exam table C = movable C-arm D = monitor screen E = ultrasound F = ventilator
This setup was installed at Osaka General Medical Center in Japan nearly 10 years ago. The authors have written occasional papers about it, and have now performed a study on its impact on trauma patient survival. They studied major trauma patients during two time periods. The first was pre-installation (2007-2011), and the second started immediately after installation (2011-2020). They specifically looked at 28-day mortality, and tried to tease out the relation to injury severity.
Here are the factoids:
- About a thousand patients were studied, 348 in the pre (conventional) group and 702 in the post (hybrid) group
- 28-day mortality was significantly lower in the hybrid group
- Using a fancy statistical test (cubic spline analysis), they showed that 28-day mortality sharply decreased 200 days after installation of the hybrid ER
- Mortality decreased disproportionately more in the hybrid ER as the injury severity score (ISS) increased
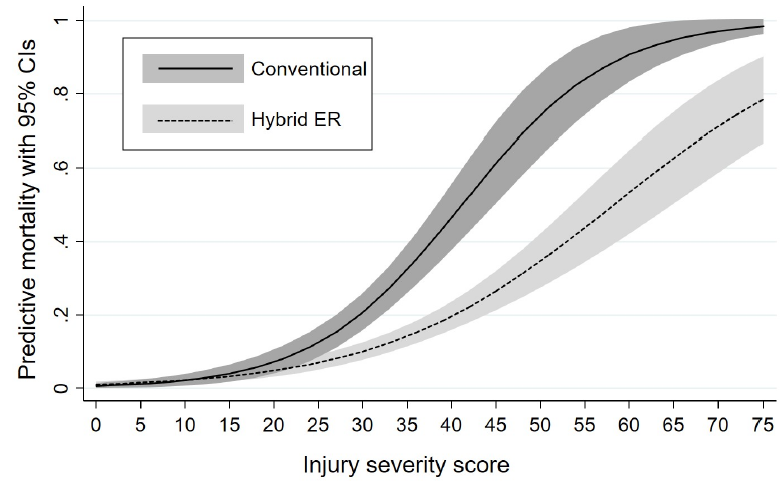
The authors concluded that the hybrid ER may have improved survival, especially in the more severely injured patients.
Here are my comments: Hmm. This is an association study that only looks at one variable, the new hybrid ER room. How many other variables may have a potential impact on survival? And how have those variables changed over the past 11 years? I worry that the study premise is too simplistic, but it certainly makes this unique resource look good.
Here are some questions for the presenter and authors:
- How did you select your patients? You describe about 1,000 patients over 11 years, which is only about 100 per year. What about all the others?
- What is it about the hybrid room that you think confers such a survival benefit to your patients? It seems to work for all patients, blunt or penetrating, badly hurt or not. What’s the magic?
- Do you see the same effect for patients who were treated at other hospitals first and then transferred? The extra time that passed could decrease survival in severely injured patients.
- Please explain cubic spline analysis clearly. I always worry when super-fancy statistical tests are needed to detect a difference. Why was it needed in this case?
- Why did it take 200 days to see an effect from the installation of the hybrid ER? What happened at that point in time?
- Please explain how the actual survival is so much better than predicted for ISS=75 patients. Your graph shows an actual survival of about 22%, as opposed to the 3% in your conventional ER. That is a massive improvement! How do you do it?
As you can see, I’m a bit uncertain about how this works and how the lessons can be applied to other centers. This is a unique resource, and the rest of the world needs to know a lot more about it before deciding to try it out themselves.